Abstract
Previously it has been found that an important risk buffering strategy in the Saami reindeer husbandry in Norway is the accumulation of large herds of reindeer as this increases long-term household viability. Nevertheless, few studies have investigated how official policies, such as economic compensation for livestock losses, can influence pastoral strategies. This study investigated the effect of received predation compensation on individual husbandry units’ future herd size. The main finding in this study is that predation compensation had a positive effect on husbandry units’ future herd size. The effect of predation compensation, however, was nonlinear in some years, indicating that predation compensation had a positive effect on future herd size only up to a certain threshold whereby adding additional predation compensation had little effect on future herd size. More importantly, the effect of predation compensation was positive after controlling for reindeer density, indicating that for a given reindeer density husbandry units receiving more predation compensation performed better (measured as the size of future herds) compared to husbandry units receiving less compensation.
Similar content being viewed by others
Introduction
Nomadic pastoralists (cf. Næss et al. 2009 for a definition) inhabit marginal areas characterized by large temporal stochasticity in environmental conditions which have the potential of influencing animal survival (Behnke 2000; Mace 1991; Behnke and Scoones 1993; Coulson et al. 2001; Coulson et al. 2000; DelGiudice et al. 2002; Patterson and Messier 2000). In Norway, for example, the environment is characterized by year-to-year variation in environmental conditions that can potentially have a dramatic effect on the reindeer husbandry (Tveraa et al. 2007; Bjørklund 1990). The reindeer husbandry is subject to periods of both decrease and increase in reindeer numbers where populations are limited by a combination of negative density dependence and winter climatic conditions (Tveraa et al. 2007) affecting survival and reproduction through individual body reserves (e.g., Tveraa et al. 2003; Bårdsen et al. 2009; Bårdsen et al. 2008). This temporal stochasticity in environmental conditions may represent a significant risk for pastoralists (cf. Næss 2009; Næss and Bårdsen 2010).
A significant challenge facing the Saami reindeer husbandry, both from a governmental management perspective and from the perspective of individual herders, is the perceived notion that there are too many reindeer in Finnmark. Consequently, the management of the reindeer husbandry is explicitly directed towards a reduction in the number of reindeer in Finnmark so as to “[…] achieve a sustainable balance between pasture resources and reindeer density […]” (Ulvevadet 2008:61). The raison d’être for this goal can be found in the dramatic decline of lichen biomass in fall and winter pastures from the early 1970s up to around the beginning of the new millennium (Riseth et al. 2004:74; see Bråthen et al. 2007 for effects on the vascular plant community on the summer pastures). This decline has arguably been caused by a dramatic increase in reindeer numbers during the 1980s from 90,000 animals in 1976 to 210,000 in 1988 (Riseth et al. 2004:74). Consequently, the Norwegian Government has initiated several economic incentives and subsidies (Riseth and Vatn 2009:87) with the explicit aim of developing a sustainable reindeer husbandry and stimulating reindeer herders to slaughter as many reindeer as possible (Anonymous 2008a:51; Anonymous 2007:6). In spite of this, the number of reindeer has increased in recent years and one reason for this overall increase may be related to pastoral risk management (Næss and Bårdsen 2010). This again provides a partial explanation for why previous studies have found that Saami reindeer herders use a substantial amount of cooperative labor effort to increase herd size (Næss et al. 2010; Næss et al. 2009).
Predation Risk and Predation Compensation
Predatory species compete with humans for the use of resources such as livestock. Consequently, predation can cause significant damages to human livelihoods and thereby raise human-wildlife conflicts (Schwerdtner and Gruber 2007:354; Wang and Macdonald 2006). Predation may thus be an important risk for pastoral production as livestock depredation caused by predation may have significant economic consequences for pastoralists (cf. Butler 2000; Mishra 1997; Schwerdtner and Gruber 2007:354; Wang and Macdonald 2006). For example, Patterson et al. (2004:507) estimated livestock predation to represent 2.6% of the herd’s economic value among Kenyan ranchers. Furthermore, Butler (2000:23) recorded economic loss averaging 12% of each household’s net annual income for pastoralists in Zimbabwe, while Oli et al. (1994, cited in Butler 2000) estimated in Nepal that livestock loss due to predation from snow leopards (Panthera uncia) represented a quarter of the average per capita income.
Consequently, an important tool for managing human-wildlife conflicts is damage compensation schemes, which distribute the costs between those who benefit from conservation and those who suffer the costs of damage (Wang and Macdonald 2006; Nyhus et al. 2003; Schwerdtner and Gruber 2007:354; Fourli 1999). In Norway, this compensation is received after the damage has occurred and the amount of compensation is based on: (1) demands made by the reindeer owners; (2) the number of documented kills (usually a very low percentage of the demands made by the owners); and (3) the number of kills seemed likely by the government (a somewhat subjective judgment, see Anonymous 2001b; Anonymous 2001a:§7 & 8 for more details). It has been argued that a serious problem with such a compensatory scheme (i.e., compensation after the damage has occurred) arises from asymmetric information about the actual damage costs, i.e., since the victim of the damage reports the case to the responsible body, there is an incentive to over-report damages to gain additional income (Schwerdtner and Gruber 2007:356).
Furthermore, Bulte and Rondeau (2005:17) have argued that predation compensation programs may alter the optimal number of livestock kept by pastoralists: without compensation, an important cost connected to increasing the number of livestock is the probability that additional animals will be lost to predators. Consequently, while predation may reduce the profitability of raising livestock, or at least the profitability of increasing herd size (Bulte and Rondeau 2005) as livestock owners have to invest resources in order to be able to increase their herd size (Næss et al. 2010; Næss et al. 2009), “[w]ith the risk of predation covered by compensation, it is optimal to increase the stocking rate” (Bulte and Rondeau 2005:17).
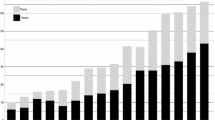
On a national scale, predation compensation in the reindeer husbandry in Norway has almost doubled in a 3 year period: in 2004 the compensation was 22,414,000 NOKFootnote 1 while by 2007 it had increased to 43 020 000 NOK (Anonymous 2008b:31). Furthermore, the Norwegian Government only compensates a fraction of the reported losses, e.g., in 2006/2007, 51 749 reindeer were reported to be lost to predation but only 30% of this reported loss was compensated (1.8% of this again was documented; Anonymous 2008a:40). In light of this, the almost doubling in predation compensation from 2004 to 2007 may arguably represent enormous losses of animals due to predation experienced by reindeer herders in Norway. Consequently, the reindeer husbandry in Norway should be regulated by increased predation rates/overall loss. Nevertheless, during the same period the overall reindeer abundance also increased, setting the stage for the apparently paradoxical situation where both the amount of compensation and reindeer numbers have reached historically high levels (Fig. 1). Moreover, on a national scale the relationship between predation compensation and overall reindeer abundance has changed: before 2004 there seems to have been a negative relationship between predation compensation and abundance (Fig. 2a), while after 2004 the relationship is weakly positive, without a corresponding increase in calf production (Fig. 2b). Predation compensation may thus represent an economic input into the reindeer husbandry that may reduce the need to slaughter and subsequently increase overall reindeer abundance, even though environmental factors like winter climate (Bårdsen et al. 2009; Bårdsen et al. 2008) and density-dependence (e.g., Næss and Bårdsen 2010; Næss et al. 2009; Tveraa et al. 2007) have been shown to influence the reindeer husbandry negatively. Consequently, this paper aims at investigating how both (1) density dependence (DD; current density of animals) and (2) amount of predation compensation (PC) affects individual husbandry units’ future herd size.
Methods
Study Area
The reindeer husbandry is said to be the cornerstone of the Saami culture in Fennoscandia (Bostedt 2001). Although it is difficult to come up with accurate dating of the origin of reindeer husbandry as a pastoral economy, it developed at least 400 years ago (Riseth and Vatn 2009; Paine 1994; see e.g., Bjørklund 1990; Bostedt 2001 for other estimates) and probably evolved from a hunting culture based on wild reindeer. The newer history of Saami reindeer husbandry can be summarized as being influenced by an increased meat and market adaptation coupled with an increased sedentarization (Riseth 2006). After the Second World War the life of the Saami reindeer herders changed as herding became more an occupation than a way of life. Moreover, during the 1950s access to markets increased, and from the middle of the 1960s the reindeer husbandry underwent major technological changes with the introduction of snowmobiles and later all-terrain vehicles (Riseth and Vatn 2009).
At the end of the 1970s new steps in governmental involvement in the reindeer husbandry were initiated. Of importance here is that in 1978 a new Reindeer Management Act was adopted, which was intended to establish a framework for governance (primarily through increased co-management) that aimed at limiting the growth of both husbandry units and herds as well as making sure that reindeer herders and their representatives should be accountable for their decisions (Riseth and Vatn 2009:101; see also Ulvevadet 2008).Footnote 2 Berg (2008) has argued that the Reindeer Management Act of 1978 was one important political initiative that laid the foundation for a change into a corporative reindeer husbandry, which not only meant production of meat for subsistence and sale but also for official subsidies (see also Riseth and Vatn 2009; Ulvevadet 2008). During this period a wide array of production subsidies became available for the reindeer husbandry (Riseth and Vatn 2009).
Apart from economic subsidies aimed stimulating production, the reindeer husbandry in Norway also receives compensation for reindeer lost to predators considered to be threatened (i.e., protected by law), more specifically to lynx (Lynx lynx), wolverine (Gulo gulo), brown bear (Ursus arctos), wolf (Canis lupus) and golden eagle (Aquila chryaetos) (Anonymous 2001b:4; Anonymous 2001a:§2a). Reindeer herders have reported that predators cause ~75–95% of calf losses and ~60–85% of adult losses (Anonymous 2008a:40), and in a study measuring mortality among reindeer, predation was found to account for 65.2% of the total reindeer mortality during 15 April 1995 to 15 April 1996 (135 reindeer of 612 radio-collared reindeer were found dead with 88 deaths caused by predation, Nybakk et al. 2002). Consequently, predation compensation is arguably a close proxy for reindeer losses in the reindeer husbandry in Norway.
The explicit rationale underlying predation compensation is both to: (1) compensate loss and subsequent costs when reindeer are lost or injured by predators; and (2) reduce the loss of reindeer (Anonymous 2001b:3–4; Anonymous 2001a:§1).Footnote 3 Normally, compensation from the Norwegian Ministry of Environment will be given when reindeer are lost or have disappeared. In general terms, compensation for loss covers the direct economic loss from losing animals and is usually calculated as the expected monetary values from meat sales and concurrent production subsidiesFootnote 4 (Anonymous 2001b; Anonymous 2001a§2c). Accordingly, in many districts it has been common that half of the herders’ income has been generated by different support and compensatory arrangements (Berg 2008:189).
Study Design
Different reindeer summer pasture districts represent heterogeneous units that differ in both climate and herding strategies (e.g., Tveraa et al. 2007). Districts with temporally persistent high and low reindeer density and offspring body mass were located through latitude and longitude gradients in Finnmark, Northern Norway, which reflect a gradient in climatic conditions (see Ims et al. 2007; Bråthen et al. 2007 for details). Differences in density formed the basis of a paired-block design between neighboring districts with low and high density. This quasi-experimental design, consisting of 10 pairs and 20 districts, was adopted to separate the effects of reindeer density from other environmental factors (cf. Næss and Bårdsen 2010 for details on the study design applied in the present study). While not estimating the effects of the design directly, the present study used the same districts to ensure that the analyses were based on an unbiased subsample of heterogeneous districts.
Study Protocol
This study is based on data compiled annually by the Norwegian reindeer husbandry administration (where 31 March represents the end date for each year; see Fig. 3). The data cover the period from 2000 to 2007 where herd sizes are based on counts made by herders that are regularly checked by the authorities (Anonymous 2005a). Data pertaining to predation compensation are compiled annually by the Directorate for Nature Management (an advisory and executive agency under the Norwegian Ministry of Environment) and represent monetary compensation for reindeer losses based on reports from reindeer owners. We made a selection of husbandry units with a minimum of 70 reindeer, a relatively conservative estimate of the number of animals needed for maintaining viable herds (Næss and Bårdsen 2010; Næss et al. 2010). The dataset, which is organized at the husbandry unit level,Footnote 5 contains the following variables (Fig. 3 provides a visual representation):
- N t+1 (response):
-
A continuous (husbandry unit level) variable denoting the total spring herd size at the beginning of the next year (i.e., at t = t+1).
- t :
-
A factor variable with each year from 2000 to 2007 acting as levels.
- PredComp t-1 :
-
A continuous (husbandry unit level) variable denoting the amount of compensation received due to reindeer assumed killed by threatened or endangered predators. This variable is a proxy for loss at t-1 and measures income at t. Measured at the scale of 10 Kilo NOK (1 Kilo NOK = 1,000 NOK). We expect a nonlinear effect on future herd size as we expect that the effect of predation compensation follows the law of diminishing marginal returns consistent with general economic theory (see e.g., Katz and Rosen 1994).
- \( {D_{{aroun{d_t}}}} \) :
-
A continuous (district unit level) variable denoting the density, individuals km-2, of reindeer in the districtFootnote 6 (after subtracting the number of animals in the husbandry unit itself). This variable, thus, measures the density of animals around each husbandry unit per year.
- ID par :
-
This design variable labels each pair of districts (contacting a ‘low’ and a ‘high’ density neighboring district as described above). This is a factor variable with each par number from 1:10 acting as levels.
- ID district :
-
This design variable labels each district in study. This is a factor variable with each district number from 1:20 acting as levels.
A year in the reindeer husbandry starts the 1st of April 1 year and ends at the 31st of March the next. In relation to the variables used in this study, N t and \( {D_{{aroun{d_t}}}} \) are variables measured at the beginning of the year (t), while N t+1 is measured at the beginning of the next year (t + 1). PredComp t−1 represents the compensation received from losses due to predation experienced during the previous year (i.e. at t−1). Our analyses was organized in this way as predation compensation is received by the husbandry units with a 1 year lag (i.e. at t + 1)
Statistical Analyses
To statistically investigate how both (1) density dependence and (2) amount of predation compensation affected individual husbandry units’ future herd size we used the following models: (1) \( {N_{{t + 1}}} = {D_{{aroun{d_t}}}} \) [density dependence (DD)]; (2) N t+1 = PredComp t-1 [predation compensation (PC)]; and (3) \( {N_{{t + 1}}} = {D_{{aroun{d_t}}}} + PredCom{p_{{t - 1}}} + {D_{{aroun{d_t}}}} \times PredCom{p_{{t - 1}}} \)(DD vs. PC). Covariates, i.e.,t, \( PredComp_{{t - 1}}^2 \) and a priori considered interactions were included and excluded within the ‘paradigm’ of model selection (e.g., Burnham and Anderson 2002 & Appendix I). Statistical analyses were performed in R (R Development Core Team 2007). All tests were two-tailed and the null-hypothesis was rejected at an α-level of 0.05.
Linear mixed-effect models (lme) applied using the nlme package (Pinheiro and Bates 2000; Pinheiro et al. 2007) were used to analyze the effect of the predictors (measured in a given year, t) on the number of reindeer present one year ahead (i.e., at t + 1). The main predictors, i.e., PredComp t−1 and \( {D_{{aroun{d_t}}}} \), and the covariates were applied as fixed effects, whereas ID district nested in ID par were included as random effects. All mixed effect-models were fitted with random intercepts only (Næss and Bårdsen 2010; Næss et al. 2010 provide the rationale for this; see also Bates 2005; Bolker et al. 2009; Galwey 2006).
Our aim was to estimate three effects: (1) the main effect of the density of animals around the husbandry unit (DD & DD vs. PC); (2) the main effect of the amount of predation compensation received by the husbandry unit (PC & DD vs. PC); and (3) the interaction between these two main predictors (DD vs. PC). Consequently, these effects were kept in all candidate models based on our a priori expectations (see e.g., Burnham and Anderson 2002). From this model, we formed a pool of candidate models where all covariates and interactions were removed sequentially. Akaike’s Information Criterion (AIC) was used to assess the fit of several candidate models (e.g., Burnham and Anderson 2002; Anderson 2008), and whenever the difference in AIC (Δ i ) was <1.5 we used the simplest candidate model for inference (Appendix I). Following Pinheiro and Bates (2000), maximum likelihood fitted models were used when models were compared, whereas a restricted maximum likelihood fitted model was used for parameter estimation. We used the treatment contrast comparing each year with the baseline-level for year (2000), and Wald statistics to test the hypothesis that the contrasts were not significantly different from zero (see Pinheiro and Bates 2000 for details).
Results
Density Dependence (DD)
Reindeer density in a given year was a negative predictor of herd size the following year [main effect of \( {D_{{aroun{d_t}}}} \): -1.0272 (Table 1)]. There was a non-significant positive interaction between \( {D_{{aroun{d_t}}}} \)and year (t) during 2001–2003, while during 2004–2007 the effect of \( {D_{{aroun{d_t}}}} \) was more negative compared to 2000 (Table 1: even though not statistically significant in 2004). Clear differences in N t+1 occurred between years (main effects of t: Table 1). Moreover, N t+1 showed a clear temporal trend as it increased from 1 year to the next for all years, and during 2002–2007 N t+1 was significantly different from 2000 (Table 1 & Fig. 4). The random effects parts of the model revealed that the variability between districts (ID district ) [nested in district pairs (ID par )] was higher than the variability between ID par , indicating that a higher proportion of the variance was explained at the level of ID district than at the level of ID par . In essence, we found evidence of negative density-dependence as reindeer density in the district had a negative effect on husbandry units’ future herd size, and this negative density dependence was more prominent in some years compared to others.
Scatter plot showing herd size 1 year ahead (N t+1) as a function density of reindeer around the husbandry unit (\( {D_{{aroun{d_t}}}} \), in km−2) for 2 years (2000 and 2006). The lines show predicted values from the model presented in Table 1, whereas points show data on N t+1 and \( {D_{{aroun{d_t}}}} \)for the 2 years. Please note the difference between the districts, i.e. one of the random effects in the model, which is represented by different symbols since without taking the grouping effect into account, the relationship between N t+1 and \( {D_{{aroun{d_t}}}} \)was not clearly negative. This was confirmed by fitting a regular linear regression \( \left( {{N_{{t + 1}}} = {D_{{aroun{d_t}}}} + t + {D_{{aroun{d_t}}}} \times t} \right) \) on the whole dataset disregarding possible grouping effects, where \( {D_{{aroun{d_t}}}} \) was positive, and the same model on a subset consisting of just one district, where \( {D_{{aroun{d_t}}}} \) was negative. We interpret this discrepancy as an effect of the fact that \( {D_{{aroun{d_t}}}} \)is defined on the district level (a given value for \( {D_{{aroun{d_t}}}} \) is thus not comparable between districts)
Predation Compensation (PC)
Predation compensation was a positive predictor of herd size the following year [main effect of PredComp t−1: 0.01265 (Table 2)]. The PredComp t−1 × t interaction showed that the positive effect of PredComp t-1 increased during the study period, although this interaction was non-significant in 2007 (Table 2). The relationship between N t+1 and amount of predation compensation was also curvilinear as a negative second-order polynomial term was statistically significant (main effect of \( PredComp_{{t - 1}}^2 \): -0.00002). This indicates that a negative feedback between predation compensation and N t+1 did occur as the positive effect of predation compensation was weakened as the amount of predation compensation increased (Table 2 & Fig. 5). The strength of this second-order polynomial increased across years as a significant negative \( PredComp_{{t - 1}}^2 \times t \) interaction was present (apart from 2007: Table 2). The strength of the polynomial term was weakest in 2001 leading to an approximately linear relationship, whereas the strongest diminishing return was apparent in 2006 (when the interaction gave the strongest negative point estimate for the polynomial term; Table 2 & Fig. 5). Clear differences in N t+1 did again occur between years (main effects of t: Table 2) and as the statistically significant effects of year were positive, this analysis also supports the general increase in N t+1 over time. The random effects parts of the model revealed that the variability between ID district and ID par (where variability between districts was the most pronounced) were substantially lower than in the analysis above (comparing Tables 1 and 2). This means that the effect of predation compensation (per se) affected herding units more equally (irrespective of district membership) compared with reindeer density. In essence, we found evidence of a positive effect of predation compensation on husbandry units’ future herd size, but the effect of PredComp t−1 was temporarily variable.
Scatter plot showing herd size 1 year ahead (N t+1) as a function of the amount predation compensation received by the husbandry unit (PredComp t−1, in Kilo NOK) for 2001 and 2006. The lines show predicted values, which were curvilinear due to the effect of \( PredComp_{{t - {1}}}^2 \) and its interaction with year (t), from the model presented in Table 2, whereas points show data on N t+1 and PredComp t−1 for the 2 years
Density Dependence (DD) vs. Predation Compensation (PC)
The effects of both predation compensation and reindeer density were statistically significant and similar as in the two previous analyses [main effect of PredComp t−1: 0.00818 and main effect of \( {D_{{aroun{d_t}}}} \): -0.81405 (Table 3)]. The significant and positive effect of the \( PredCom{p_{{t - 1}}} \times {D_{{aroun{d_t}}}} \) interaction indicates that the positive effect of PredComp t−1 was increasingly positive for higher densities, and similarly that the negative effects of density were weakened for herding units receiving large amounts of predation compensation (Table 3 & Fig. 6). Both predation compensation and density interacted with year indicating that both effects were temporally variable (Table 3): (1) the strength of predation compensation increased over years (although PredComp t−1 × t (2007) was not significant); (2) there was a positive interaction between \( {D_{{aroun{d_t}}}} \)and year (t) during 2002–2004, indicating that the negative effect of \( {D_{{aroun{d_t}}}} \) was reduced during this period (not significant in 2002, and the strength of this positive interaction was weaker than the negative main effect of density). During 2005–2007 the effect of \( {D_{{aroun{d_t}}}} \) was even more negative compared to 2001 (Table 3 & Fig. 6: even though not statistically significant in 2005).
Contour plot showing herd size 1 year ahead (N t+1) as a function of the amount predation compensation received by the husbandry unit (PredComp t−1, in Kilo NOK), density of reindeer around the husbandry unit (\( {D_{{aroun{d_t}}}} \), in km−2) and the interaction between them for 2000 (a) and 2006 (b). The lines show predicted values, which were curvilinear due to the effect of \( PredComp_{{t - {1}}}^2 \), and its interaction involving year (t), from the model presented in Table 3, whereas points show data on \( {D_{{aroun{d_t}}}} \) and PredComp t−1 for the 2 years
Consequently, even though the effects of both PredComp t−1 and \( {D_{{aroun{d_t}}}} \)were temporary variable, i.e., both stronger and weaker than in 2001, the direction of both effects was similar to that found in 2001. As in the PC analysis above the relationship between N t+1 and predation compensation showed a marginal curvilinear relationship [main effect of \( PredComp_{{t - 1}}^2 \): -0.00001 (Table 3 & Fig. 6)]. The effect of \( PredComp_{{t - 1}}^2 \) was at its weakest in 2001 since all interactions with year were negative (Table 3: all significant except for the 2004 interaction). Moreover, a robust diminishing return was apparent in 2006 [i.e., the \( PredComp_{{t - 1}}^2 \times t \)(2006) interaction was strongest], where the strong negative interaction strengthened the negative effect of \( PredComp_{{t - 1}}^2 \) (Table 3). The random effects parts of the model revealed that the variability between ID district and the variability between ID par were similar as in the previous analysis where \( {D_{{aroun{d_t}}}} \) was included in the fixed effects part of the model: ID par explained less variance compared to ID district . In essence, we found evidence of negative density-dependence and a positive effect of predation compensation on husbandry units’ future herd size.
Discussion
The main finding in this study was that predation compensation had a positive effect on husbandry units’ future herd size. The effect of predation compensation, however, was nonlinear, indicating that predation compensation had a positive effect on future herd size only up to a certain threshold after which adding additional predation compensation had little effect on future herd size (i.e., a negative feedback or a diminishing marginal return). Additionally, we found that reindeer density had a negative effect on the husbandry units’ future herd size. Importantly, we also found that negative density dependence acted together with the positive effect of predation compensation, indicating that husbandry units can at least to some degree compensate for negative density dependence through predation compensation.
Density Dependence (DD)
In line with Næss and Bårdsen (2010), this study found that density around a husbandry unit had a negative effect on the unit’s future herd size. This effect varied between years, but not to such a degree that the relationship went from negative to positive.
Predation Compensation (PC)
This study also found that predation compensation had a positive effect on future herd size. This effect, however, was nonlinear and characterized as a negative feedback (a diminishing marginal return) as predation compensation had a positive effect on future herd size up to a certain threshold after which adding more predation compensation had a diminishing effect on future herd size. It should be noted that the effect of diminishing marginal returns varied between years, and in some years it was so small as to be approximately linear.
Density Dependence (DD) vs. Predation Compensation (PC)
When both density and predation compensation were assessed simultaneously we found that negative density dependence acted together with the positive effect of predation compensation. More importantly, the effect of predation compensation on future herd size was positive after controlling for density, indicating that for a given density of reindeer, husbandry units receiving comparably more predation compensation were doing better (measured as the size of future herds) than husbandry units receiving less. This point was substantiated by the positive interaction between predation compensation and density, which showed that: (1) the positive effect of predation compensation was increasingly positive for higher densities; and (2) negative density dependence was weakened for large amounts of predation compensation.
Large Herds as a Risk Reducing Strategy
The accumulation of animals as a buffer against future loss has been argued to be an efficient strategy for countering risks (see e.g., Hjort 1981; Templer et al. 1993; Næss 2009). This idea has been corroborated by e.g., McPeak (2005), who found that for Gabra pastoralists (Northern Kenya) a larger pre-crisis herd size was correlated with larger post-crisis herd size (see also Fratkin and Roth 1990; Roth 1996). For the Saami reindeer husbandry (Norway), Næss and Bårdsen (2010) found that large herd size maximizes long term viability for pastoral households since husbandry units with large herds were on average larger the following year relative to smaller ones. This effect was apparent during periods of both overall increase and decrease in reindeer abundance.
While predation may reduce the profitability of increasing herd size, with the risk of predation covered by compensation it may be optimal to increase the number of livestock (Bulte and Rondeau 2005:17). As large herd size maximizes long term viability for pastoral households, the results from this study may be taken to indicate that predation compensation may represent an additional risk reducing strategy in the reindeer husbandry. While the underlying mechanisms for this may not be easily apparent, it could be argued that predation compensation may represent a viable income for reindeer herders. Income generated from governmental initiatives such as predation compensation may make it possible for herders to reduce slaughtering (see e.g. Nilsen and Mosli 1994; Riseth 2000, 2003; Riseth and Vatn 2009) and convert otherwise slaughtered animals to livestock capital, which again has a positive impact on long term livestock viability. Our finding that predation compensation 1 year is positively related to future herd size indicates that this sort of compensation may act as an incentive for the herders to increase their herd size, which ultimately leads to regional increase in reindeer numbers.
Problems with Confounding and Possible Solutions
All observational studies have potential problems in relation to confounding which may lead to spurious relationships between the included predictors and the response and to biased estimation of effects (Cohen et al. 2003). Problems in relation to confounding were, however, reduced as we had a priori expectations that formed the basis for the set of candidate models from which we selected the most parsimonious model and used this model for inference (in accordance with the information-theoretic approach, see Burnham and Anderson 2002).
Nevertheless, there may be several important factors influencing future herd size not accounted for in our analyses. First, it could be argued that if predation compensation is a proxy for real losses, one should expect that predation compensation 1 year should be negatively related to herd size 1 year ahead: (1) as the compensation increases, individual husbandry units should experience increasing losses; which (2) should affect future herd size negatively.Footnote 7 Nevertheless, this study has not explicitly incorporated a measure of herd reproduction in the analyses. It may, for example, be argued that loss, and consequently compensation, may be positively related to future herd size as long as the birth rate exceeds the death rate. Several solutions to this problem exist: (1) replace future herd size (N t+1) with the growth rate (N t+1/N t ) as a response; (2) include the number of calves (or some other measure of reproduction) as a covariate in the analyses as this would provide a measure of how compensation and loss influence future herd size after statistically controlling for reproduction. We decided to use herd size rather than growth rate as a response because: (1) the importance of herd size as a measure of pastoral performance is widely documented in the literature (cf. Næss and Bårdsen 2010; Næss 2010; see also Bollig and Göbel 1997; Coughenour et al. 1985; Hjort 1981; Fratkin and Roth 1990; Roth 1996; Templer et al. 1993); and (2) because the growth rate is more difficult to interpret than herd size (Appendix II). Adding the number of calves as a covariate was not a good solution as this variable was strongly correlated with predation compensationFootnote 8 resulting in colinearity problems (see Appendix II, and Table 7a).
Another option would be to include present herd size as a covariate. Nevertheless, present herd size and predation compensation were correlated (Appendix II, Table 7b). Since Næss and Bårdsen (2010) found that present herd size was a good predictor for future herd size it could be argued that predation compensation represented a proxy for herd size. In other words, the positive correlation between present herd size and predation compensation can arguably indicate that it is present herd size and not predation compensation that is responsible for the results presented in this study.
Important factors such as present herd size and number of calves could thus not be statistically controlled for due to problems related to collinearity (Appendix II). We were left with a choice whether to include the predictors we had theoretical expectations to (i.e., predation compensation) or to replace them with other possible important covariates (e.g., present herd size). As decisions regarding which predictors to include or exclude cannot be assessed statistically, this decision needs to be based on theoretical considerations relevant to the hypotheses tested (Berry and Feldman 1985; Licht 1995). Consequently, we chose to focus on predation compensation because this was the variable we had an a priori interest in estimating. Moreover, predation compensation represents a distinct theoretical concept measuring both reindeer loss and economic input. We tried to test some of the issues related to collinearity by making a new variable measuring the number of calves produced per reindeer, a variable that greatly reduced problems pertaining to collinearity (Appendix II: Table 7c). Based on this finding we re-fitted the best models using the number of calves produced per reindeer as a covariate. These analyses revealed that while the number of calves per reindeer was a statistically significant positive predictor for future herd size, the presence of this predictor changed neither the original results nor the interpretation of them (see Appendix II: Tables 8, 9, 10 for details). This indicates that the results were not sensitive to confounding with the aforementioned variables not included in the analyses.
Livestock and Predation Worldwide
To put things in perspective, annual predator off-take rates among pastoralists’ herds have been found to range between 0.8% and 12% in different parts of the worldFootnote 9: for example, in Ladakh, India, one study found that half of the households lost 1–12% of their livestock to predators over a 14-month period (Jackson et al. 2003). For the Maasai, Northern Tanzania, Kissui (2008:7) found an annual loss to predation of 1% for cattle and 4% loss for goats and sheep. As for pastoralists in Niger, McShane & Grettenberger (1984, cited in Butler 2000:29) estimated that 2.1% of goats and 0.5% of sheep were lost to predation. Furthermore, Mishra (1997:339) found that in the Indian state of Himachal Pradesh 1.6 livestock heads/family/year, representing an annual loss of 12% of the livestock holding, were reported lost due to predation, and Oli et al. (1994, cited in Butler 2000) estimated in Nepal that snow leopards killed 2.6–5.1% of total livestock holdings.
As for the reindeer husbandry in North-Trøndelag, Norway—an area known to hold a high density of large carnivores (especially lynx)—predation resulted in a loss of 14.4% during a 1 year period (Nybakk et al. 2002). From 2006 to 2009 in Finnmark, the amount of reindeer reported lost to predators and for which compensation was requested represented an average of 24.1% (SD ± 2.3%, number of husbandry units ranged from 389 to 398 in the same period) of the total number of reindeer at the beginning of the year (reindeer numbers from: Anonymous 2009b:8, Anonymous 2010a:8, and number of reindeer reported lost to predators from: Anonymous 2010b). Compared to other areas worldwide, loss due to predation seems thus to be high in Finnmark. Moreover, the number of predators in Finnmark seems to be lower than other parts of Norway, e.g., the average number of lynx family groups during 2000–2003 was 2.5 in Finnmark and 15.0 for the middle part of Norway,Footnote 10 while the number of breeding wolverines was 4.5 in Finnmark and 15.5 for the middle parts of Norway during the same period (lynx and wolverines accounted for 41.06% and 29.21% of the reported losses in 2008 on a national scale: Anonymous 2010; 2003).Footnote 11
Concluding Remarks and Management Implications
This paper investigated the relative importance of possible density-dependence and predation compensation as a governmental management initiative on husbandry units’ future herd size. While a previous study found support for the general assumption that large herds may be an efficient risk reducing strategy for nomadic pastoralists (cf. Næss and Bårdsen 2010), the same study also hypothesized that that governmental initiatives such as predation compensation may positively interact with the risk beneficial aspects of having a large herd. Loss of animals due to predation can thus be viewed as being subsidized by the Norwegian Government (Anonymous 2008a).
The present study might shed some light on why both the abundance of reindeer and predation compensation have recently reached an all time high within the Norwegian reindeer husbandry. Since losses are based on self-reporting, predation compensation can arguably present an incentive for reindeer herders to over-report losses (see also Schwerdtner and Gruber 2007). The beneficial aspect of having a large herd in such a subsidized system is self-evident: having a large herd gives reindeer husbandry units the possibility to report much larger losses than husbandry units with comparable smaller herds. Moreover, predation compensation may represent a viable income for reindeer herders apart from marketing meat for sale through slaughtering. This is in line with other studies which have argued that the more money the household receives from sources such as subsidies, the less they need to slaughter reindeer for marketing (e.g., Riseth and Vatn 2009:100-101). Additionally, for reindeer herders it can be argued that the value of a given reindeer is higher when it is reported lost to predation than it is by being slaughtered as reporting it lost to predation can be done without actually losing the animal. Such over-reporting can be a rational strategy for gaining additional value from their herds of reindeer.Footnote 12 The rationale for this is self-evident: first, by slaughtering a reindeer for sale the reindeer’s value is simply the fixed monetary value received from the slaughterhouse. Second, by reporting a live animal lost to predation, the potential value of the animal increases—the value of the animal is then not simply the predation compensation received for the animal (which varies according to animal class and district, see Anonymous 2009 for details),Footnote 13 but also the value received from e.g., slaughtering the animal privately. Of special importance here is that a live animal reported lost has a clear risk beneficial value: predation compensation may reduce the need for herders to slaughter animals to get money, i.e., they can convert otherwise slaughtered reindeer to livestock capital and thereby increase herd size. As Næss and Bårdsen (2010) found that large herd size maximizes long term viability for pastoral households since husbandry units with large herds are performing better relative to those with smaller herds, predation compensation may represent an additional tool for reindeer herders to attain this goal. Consequently, present governmental management initiatives aimed at reducing herd size have to change so as to incorporate factors associated with risk reducing strategies in their management plans. Presently, management initiatives may be viewed as being based on a faulty assumption that there is a correspondence between the objectives of reindeer herders and governmental plans, e.g., increased meat production efficiency. Results from this study and from Næss and Bårdsen (2010) indicate that reindeer herders may be more concerned with maximizing long term livestock viability by accumulating animals. In closing, it can be argued that “[s]uggested ‘improvements’ to management practices that increase productivity may not be sustainable because they reduce household viability” (Mace and Houston 1989:186).
Notes
100 NOK = $17.11 per 26.01.2010.
This focus on co-management has been broadened in the Reindeer Management Act of 2007 (Ulvevadet 2008:66).
The reindeer owners do need to fulfill certain conditions in order to receive compensation: husbandry units (i) have to have acted with care and taken necessary precautions to avoid or reduce loss; (ii) acted in accordance with the Reindeer Management Act and the Animal Welfare Act; (iii) made sure that losses have been reported as early as possible; (iv) made sure that officially funded preventive measures have been implemented; and (v) have given correct and necessary information to substantiate the claim for compensation (Anonymous 2001a:§4).
More specifically, the calculation of the compensation rate is based on the product of: (1) average carcass body mass for the previous 3 years (per reindeer husbandry district); and (2) an index regulated price per kg meat delivered to slaughterhouses (Anonymous 2009a §2).
Formal management units consisting of several husbandry units with clear geographic delineation where reindeer husbandry is practiced. This study is based on summer districts, i.e. areas with geographic delineation during summer (see Næss 2009; Næss and Bårdsen 2010; Næss et al. 2010; Næss et al. 2009 for details).
Alternatively, predation compensation 1 year should have no effect on future herd size as the compensation may be simply used to replace lost animals.
This positive correlation may be due to the fact the larger number of calves that reindeer herders report as produced, the larger number they can report as lost to predation and thereby increase compensation. Not surprisingly, calves represent the largest percentage of loss experienced in Finnmark, e.g., during 2006/2007 calf loss represented 71.5% of the total reported loss (Anonymous 2010b).
Graham et al. (2005) found, however, in a review of livestock predation worldwide that reported losses ranges from 0.02 to 2.6% per year.
Counties included in this region: Parts of Hedmark, Oppland, Nordland and Møre og Romsdal, whole of South-Trøndelag and North-Trøndelag (Anonymous 2003).
The situation, however, seems to have changed recently where Finnmark has experienced an increase in the number of breeding wolverines (14 in 2008) and lynx (9 in 2008, Brøseth 2009). This increase, however, is partly due to the fact that the number for Troms County has been incorporated into the estimate for Finnmark in recent years.
While interviewing a representative from the reindeer husbandry administration two of the authors was shown several examples where husbandry units had reported a number of calves lost to predation that was higher than the actual number of calves the husbandry could have produced as reindeer only give birth to one offspring per year. While anecdotal in nature, this example illustrates the fact that losses may not reflect actual numbers.
For example, in the year 2008/2009 one calf was valued at average of 1222.23 ± 157.44 SD NOK, one bull at 2388.65 ± 361.15 SD NOK, one female at 1884.49 ± 262.62 SD NOK and a reindeer used for transport at 7000 ± 0 SD NOK (N = 75 districts Anonymous 2009:§1).
Note, however, that it has been argued that since collinearity is present to some degree in all observational studies the most reasonable course when facing it is to recognize it and live with the consequences (Berry and Feldman 1985:49). Nevertheless, since the models were sensitive for covariates and interactions that were included/excluded (when both number of calves and predation compensation were included), we decided to focus on the predictor which we were interested in estimating.
References
Anderson, D. R. (2008). Model Based Inference in the Life Sciences: a Primer on Evidence. Springer Science, New York.
Anderson, D. R., Burnham, K. P., and Thompson, W. L. (2000). Null hypothesis testing: Problems, prevalence, and an alternative. Journal of Wildlife Management 64(4): 912–923.
Anderson, D. R., Burnham, K. P., Gould, W. R., and Cherry, S. (2001). Concerns about finding effects that are actually spurious. Wildlife Society Bulletin 29(1): 311–316.
Anonymous. (2001a). FOR 2001-05-04 nr 468: Forskrift om erstatning for tap og følgekostnader når tamrein blir drept eller skadet av rovvilt. in hefte 6: Miljøverndepartementet, Avdeling for naturforvaltning.
Anonymous. (2001b). Rundskriv juni 2001 Viltloven: Erstatning for tap av tamrein. pp. 15: Direktoratet for naturforvaltning.
Anonymous. (2003). St.meld. nr. 15 (2003–2004): Rovvilt i norsk natur. pp. 134: Miljøverndepartementet.
Anonymous. (2005a). Ressursregnskap for reindriftsnæringen. Reindriftsforvaltningen, p. 133.
Anonymous. (2005b). Totalregnskap for reindriftsnæringen. Reindriftsforvaltningen p. 141.
Anonymous. (2007). St.prp. nr. 74: Om reindriftsavtalen 2007/2008, om dekning av kostnader vedrørende radioaktivitet i reinkjøtt, og om endringer i statsbudsjettet for 2007 m.m.. pp. 36: Det Kongelige Landbruks- og Matdepartement.
Anonymous. (2008a). Ressursregnskap for reindriftsnæringen. Reindriftsforvaltningen, p. 159.
Anonymous. (2008b). Totalregnskap for reindriftsnæringen. Reindriftsforvaltningen p. 126.
Anonymous. (2009a). FOR 2009-07-03 nr 970: Forskrift om grunnsatser for tap av tamrein drept eller skadet av rovvilt reindriftsåret 2008/2009. in hefte 9. http://www.lovdata.no/cgi-wift/ldles?doc=/sf/sf/sf-20090703-0970.html: Miljøverndepartementet, Direktoratet for naturforvaltning (in Norwegian).
Anonymous. (2009b). Ressursregnskap for reindriftsnæringen, p. 146.
Anonymous. (2010a). Ressursregnskap for reindriftsnæringen, p. 143.
Anonymous. (2010b). Rovbase 3.0. The Directorate for Nature Management. Accessed: 27.09.2010. http://dnweb10.dirnat.no/Rovbase30Innsyn/Contentpages/InnsynForsiden.aspx.
Bårdsen, B.-J., Fauchald, P., Tveraa, T., Langeland, K., Yoccoz, N. G., and Ims, R. A. (2008). Experimental Evidence of a Risk-Sensitive Reproductive Allocation in a Long-Lived Mammal. Ecology 89(3): 829–837.
Bårdsen, B.-J., Fauchald, P., Tveraa, T., Langeland, K., and Nieminen, M. (2009). Experimental Evidence of Cost of Lactation in a Low Risk Environment for a Long-Lived Mammal. Oikos 118: 837–852.
Bates, D. M. (2005). Fitting Linear Mixed Models in R. R News 5: 27–30.
Behnke, R. H. (2000). Equilibrium and Non-equilibrium Models of Livestock Population Dynamics in Pastoral Africa: Their Relevance to Arctic Grazing Systems. Rangifer 20(2): 141–152.
Behnke, R. H., and Scoones, I. (1993). Rethinking Range Ecology: Implications for Rangeland Management in Africa. In R. H. Behnke, I. Scoones, and C. Kerven (eds.), Range Ecology at Disequilibrium. New Models of Natural Variability and Pastoral Adaptation in African Savannas, Overseas Development Institute, London, pp. 1–30.
Berg, B. Å. (2008). Utviklingen av reindriften i nordre Nordland 1750–2000. In B. Evjen and L. I. Hansen (eds.), Nordlands kulturelle mangfold: etniske relasjoner i historisk perspektiv, Pax, Oslo, pp. 151–191.
Berry, W. D., and Feldman, S. (1985). Multiple Regression in Practice. Quantitative Applications in the Social Sciences 50. Sage Publications, Beverly Hills.
Bjørklund, I. (1990). Sami Reindeer Pastoralism as an Indigenous Resource Management System in Northern Norway: A Contribution to the Common Property Debate. Development and Change 21: 75–86.
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulson, J. R., Stevens, M. H. H., and White, S. S. (2009). Generalized Linear Mixed Models: A Practical Guide for Ecology and Evolution. Trends in Ecology & Evolution 24: 127–135.
Bollig, M., and Göbel, B. (1997). Risk, Uncertainty and Pastoralism: An Introduction. Nomadic Peoples 1(1): 5–21.
Bostedt, G. (2001). Reindeer Husbandry, the Swedish Market for Reindeer Meat, and the Chernobyl Effects. Agricultural Economics 26(3): 217–226.
Bråthen, K. A., Ims, R. A., Yoccoz, N. G., Fauchald, P., Tveraa, T., and Hausner, V. H. (2007). Induced Shift in Ecosystem Productivity? Extensive Scale Effects of Abundant Large Herbivores. Ecosystems 10(5): 773–789.
Brøseth, H. (2009). Bestandsstatus gaupe, jerv, ulv og bjørn fra det nasjonale overvåkingsprogrammet for rovvilt NINA 3.
Buckland, S. T., Burnham, K. P., and Augustin, N. H. (1997). Model selection: An integral part of inference. Biometrics 53(2): 603–618.
Bulte, E. H., and Rondeau, D. (2005). Why Compensating Wildlife Damages may be Bad for Conservation. Journal of Wildlife Management 69(1): 14–19.
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Springer, New York.
Butler, J. R. A. (2000). The Economic Costs of Wildlife Predation on Livestock in Gokwe Communal Land, Zimbabwe. African Journal of Ecology 38(1): 23–30.
Cohen, J., Cohen, P., West, S. G., and Aiken, L. S. (2003). Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences, 3rd ed. Lawrence Erlbaum, Mahwah.
Coughenour, M. B., Ellis, J. E., Swift, D. M., Coppock, D. L., Galvin, K., McCabe, J. T., and Hart, T. C. (1985). Energy Extraction and Use in a Nomadic Pastoral Ecosystem. Science 230(4726): 619–625.
Coulson, T., Milner-Gulland, E. J., and Clutton-Brock, T. (2000). The Relative Roles of Density and Climatic Variation on Population Dynamics and Fecundity Rates in Three Contrasting Ungulate Species. Proceedings Of The Royal Society Of London Series B-Biological Sciences 267(1454): 1771–1779.
Coulson, T., Catchpole, E. A., Albon, S. D., Morgan, B. J. T., Pemberton, J. M., Clutton-Brock, T. H., Crawley, M. J., and Grenfell, B. T. (2001). Age, Sex, Density, Winter Weather, and Population Crashes in Soay Sheep. Science 292(5521): 1528–1531.
DelGiudice, G. D., Riggs, M. R., Joly, P., and Pan, W. (2002). Winter Severity, Survival, and Cause-Specific Mortality of Female White-Tailed Deer in North-Central Minnesota. Journal of Wildlife Management 66(3): 698–717.
Fourli, M. (1999). Compensation for Damages Caused by Bears and Wolves in the European Union. European Communities.
Fox, J. (1991). Regression diagnostics. Quantitative applications in the social sciences, Sage, Newbury Park, Calif.
Fratkin, E., and Roth, E. A. (1990). Drought And Economic Differentiation Among Ariaal Pastoralists Of Kenya. Human Ecology 18(4): 385–402.
Galwey, N. (2006). Introduction to Mixed Modelling Beyond Regression and Analysis of Variance. Wiley, Chichester.
Graham, K., Beckerman, A. P., and Thirgood, S. (2005). Human-Predator-Prey Conflicts: Ecological Correlates, Prey Losses and Patterns of Management. Biological Conservation 122(2): 159–171.
Grandin, B. E. (1983). The importance of wealth effects on pastoral production: A rapid method for wealth ranking. (eds.), Pastoral systems research in sub‐Saharan Africa: proceedings of the IDRC/ILCA workshop held at ILCA, Addis Ababa, Ethiopia 21 to 24 March, 1983, ILCA, Addis Ababa, Ethiopia, pp. 237–262.
Hjort, A. (1981). Herds, trade and grain: pastoralism in a regional perspective. In Galaty, J. G., Salzman, P. C., and Choiunard, A. (eds.), The Future of Pastoral Peoples: Proceedings of a Conference Held in Nairobi, Kenya, 4–8 August 1980. International Development Research Centre, Ottawa, pp. 97–103.
Ims, R. A., Yoccoz, N. G., Bråthen, K. A., Fauchald, P., Tveraa, T., and Hausner, V. (2007). Can reindeer Overabundance Cause a Trophic Cascade? Ecosystems 10(4): 607–622.
Jackson, R., Hillard, D., and Wangchuk, R. (2003). Encouraging Local Participation in Efforts to Reduce Livestock Depredation by Snow Leopard and Wolf in Ladakh, India. Carnivore Damage Prevention News 4: 2–6.
Katz, M. L., and Rosen, H. S. (1994). Microeconomics, 2nd ed. The Irwin series in economics, Irwin, Burr Ridge, Ill.
Kissui, B. M. (2008). Livestock Predation by Lions, Leopards, Spotted Hyenas, and Their Vulnerability to Retaliatory Killing in the Maasai Steppe, Tanzania. Animal Conservation:1–11.
Licht, M. H. (1995). Multiple regression and correlation. In Grimm, L. G., and Yarnold, P. R. (eds.), Reading and Understanding Multivariate Statistics. American Psychological Association, Washington, D.C., pp. 19–64.
Mace, R. (1991). Conservation Biology - Overgrazing Overstated. Nature 349(6307): 280–281.
Mace, R., and Houston, A. (1989). Pastoralist Strategies for Survival in Unpredictable Environments: A Model of Herd Composition that Maximises Household Viability. Agricultural Systems 31(2): 185–204.
McPeak, J. (2005). Individual and Collective Rationality in Pastoral Production: Evidence from Northern Kenya. Human Ecology 33(2): 171–197.
Mishra, C. (1997). Livestock Depredation by Large Carnivores in the Indian trans-Himalaya: Conflict Perceptions and Conservation Prospects. Environmental Conservation 24(4): 338–343.
Næss, M. W. (2009). Pastoral Risk Management - The Importance of Cooperative Production. Philosophiae Doctor, University of Tromsø, Norway.
Næss, M. W. (2010). Contradictory Evidence as a Guide for Future Research - Investigating the Relationship between Pastoral Labour and Production. Nomadic Peoples 14(1): 51–71.
Næss, M. W., and Bårdsen, B.-J. (2010). Environmental Stochasticity and Long-Term Livestock Viability-Herd-Accumulation as a Risk Reducing Strategy. Human Ecology 38(1): 3–17.
Næss, M. W., Fauchald, P., and Tveraa, T. (2009). Scale Dependency and the “Marginal” Value of Labor. Human Ecology 37(2): 193–211.
Næss, M. W., Bårdsen, B.-J., Fauchald, P., and Tveraa, T. (2010). Cooperative pastoral production - the importance of kinship. Evolution and Human Behavior 31(4): 246–258.
Nilsen, R., and Mosli, J. H. (1994). Inn fra vidda: hushold og økonomisk tilpasning i reindrifta i Guovdageaidnu 1960–1993. NORUT samfunnsforskning rapport, Bajos (in Norwegian), Guovdageaidnu.
Nybakk, K., Kjelvik, A., Kvam, T., Overskaug, K., and Sunde, P. (2002). Mortality of Semi-domestic Reindeer Rangifer Tarandus in Central Norway. Wildlife Biology 8(1): 63–68.
Nyhus, P. J., Fischer, H., Madden, F., and Osofsky, S. (2003). Taking the Bite out of Wildlife Damage. Conservation in Practice 4(2): 37–40.
Paine, R. (1994). Herds of the Tundra: A Portrait of Saami reindeer pastoralism. Smithsonian series in ethnographic inquiry, Smithsonian Institution Press, Washington London.
Patterson, B. R., and Messier, F. (2000). Factors Influencing Killing Rates of White-Tailed Deer by Coyotes in Eastern Canada. Journal of Wildlife Management 64(3): 721–732.
Patterson, B. D., Kasiki, S. M., Selempo, E., and Kays, R. W. (2004). Livestock Predation by Lions (Panthera leo) and Other Carnivores on Ranches Neighboring Tsavo National Parks, Kenya. Biological Conservation 119(4): 507–516.
Pinheiro, J. C., and Bates, D. M. (2000). Mixed-Effects Models in S and S-PLUS. Statistics and Computing. Springer, New York.
Pinheiro, J. C., Bates, D. M., DebRoy, S., and Deepayan, S. (2007). nlme: Linear and Nonlinear Mixed Effects Models. R package version 3: 1–83.
R Development Core Team. (2007) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, R Development Core Team.
Riseth, J. Å. (2006). Sàmi Reindeer Herd Managers: Why Do They stay in a Low-Profit Business? British Food Journal 108(7): 541–559.
Riseth, J. Å. (2000). Sámi reindeer management under technological change 1960–1990: implications for common-pool resource use under various natural and institutional conditions: a comparative analysis of regional development paths in West Finnmark, North Trøndelag, and South Trøndelag/Hedmark, Norway. Doctor scientarium theses Norges landbrukshøgskole, Ås.
Riseth, J. Å. (2003). Sami reindeer management in Norway: modernization challenges and conflicting strategies. Reflections Upon the Co-management Alternative. In S. Jentoft, H. Minde, and R. Nilsen (eds.), Indigenous Peoples: Resource Management and Global Rights, Eburon Academic Publishers, Delft, Netherlands, pp. 229–247.
Riseth, J. Å., and Vatn, A. (2009). Modernization and Pasture Degradation: A Comparative Study of Two Sàmi Reindeer Pasture Regions in Norway. Land Economics 85(1): 87–106.
Riseth, J. Å., Johansen, B., and Vatn, A. (2004). Aspects of a Two-Pasture – Herbivore Model. Rangifer Special Issue 15: 65–81.
Roth, E. A. (1996). Traditional Pastoral Strategies in a Modern World: An example from Northern Kenya. Human Organization 55(2): 219–224.
Schwerdtner, K., and Gruber, B. (2007). A Conceptual Framework for Damage Compensation Schemes. Biological Conservation 134(3): 354–360.
Templer, G., Swift, J., and Payne, P. (1993). The Changing Significance of Risk in the Mongolian Pastoral Economy. Nomadic Peoples 33: 105–122.
Tveraa, T., Fauchald, P., Henaug, C., and Yoccoz, N. G. (2003). An Examination of a Compensatory Relationship Between Food Limitation and Predation in Semi-domestic Reindeer. Oecologia 137(3): 370–376.
Tveraa, T., Fauchald, P., Yoccoz, N. G., Ims, R. A., Aanes, R., and Hogda, K. A. (2007). What Regulate and Limit Reindeer Populations in Norway? Oikos 116(4): 706–715.
Ulvevadet, B. (2008). Management of Reindeer Husbandry in Norway - Power-Sharing and Participation. Rangifer 28(1): 53–78.
Wang, S. W., and Macdonald, D. W. (2006). Livestock Predation by Carnivores in Jigme Singye Wangchuck National Park, Bhutan. Biological Conservation 129(4): 558–565.
Zuur, A. F., Ieno, E. N., and Elphick, C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology & Evolution 1(1): 3–14.
Acknowledgments
The present study was financed by the Directorate for Nature Management; the Fram Centre, Norway and the Research Council of Norway (the FRIMUF program). Valuable comments from and discussion with Rolf A. Ims and Per Fauchald have improved the paper. We thank Hans Tømmervik for discussions and for providing additional information. We also thank one anonymous reviewer whose comments helped to improve the quality of the paper.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix I: Model Selection and the Set of Candidate Models
Selection of models used for inference in the analyses was performed within a model selection framework (e.g., Anderson et al. 2000; Buckland et al. 1997; Burnham and Anderson 2002): First, a pool of candidate models was defined. Defining the set of candidate models is an important but often underemphasized part of a statistical analysis: ‘models without biological support should not be included in the set of candidate models’ (Burnham and Anderson 2002). Thus, we kept PredComp t-1 (PC & DD vs. PC), \( {D_{{aroun{d_t}}}} \) (DD & DD vs. PC) and the interaction between them (DD vs. PC) in all analyses based on our a priori expectations (see Tables 4, 5 and 6 for details). Second, in each analysis, rescaling and ranking models relative to the value of the model with the lowest Akaike’s Information Criterion (AIC) value was performed (Burnham and Anderson 2002: Δ i denotes this difference for model i). Following Pinheiro and Bates (2000), maximum likelihood (ML) fitted models were used when these models were compared, whereas models were fitted using the restricted maximum likelihood (REML) when we extracted parameter estimates (see main text).
Appendix II: Confounding
Problems in relation to confounders was reduced as we had a priori expectations that formed the basis for the set of candidate models from which we selected the most parsimonious model and used this model for inference (see e.g., Burnham and Anderson 2002; Buckland et al. 1997; Anderson et al. 2001). This approach reduces the chances of pursuing spurious effects as the researcher limits his/her pursuit to theoretically important relationships (Burnham and Anderson 2002). This study did not explicitly incorporate a measure of reproduction (e.g., number of animals born) in the analyses. It may, for example, be argued that loss, and consequently compensation, may be positively related to future herd size as long as the birth rate exceeds the death rate.
Changing the Response – Growth Rate
This problem could be solved in a number of ways, one being to replace future herd size (N t+1) with the population growth rate (N t+1/N t ) as a response. Apart from the well documented importance of herd size as a measure of pastoral performance (see main text), the use of growth rates is problematic also because: (1) a negative relationship between compensation and growth rate would only indicate that the growth rate decreases, not that there is no growth. In other words, it is possible that compensation has a positive effect on herd size while at the same time has a negative effect on population growth rates. For example, a pastoralist may have a herd of 50 animals at t, 60 at t+1 and 70 at t+2. For all practical purposes, this herd owner does better over time because the herd size increases (and from risk point of view, increases long term livestock viability). If we use the growth rate as a response the picture changes as this shows that the herd owner is doing worse over time because the growth rate decreases from t to t+1 (r t = 1.2, r t+1 = 1.17). While the herder is doing worse, on the scale of population multiplication rate, the herd still increases and performance is better on the scale of absolute herd size. (2) Furthermore, the growth rate standardizes possible wealth differences because it does not account for wealth differences. Even though growth rates may be the same for a herd owner with 20 animals and one with 200 animals (e.g., r = 1.2), the first owner is clearly doing worse than the second (on the scale of changes in absolute herd size). When deciding how many animals to slaughter, the second herder can slaughter a larger number of animals than the first herder and still maintain viability. More to the point, the growth rate does not account for the fact that “[…] a producer with 400 animals will have different management strategies and possibilities than a producer with only 4 animals” (Grandin 1983:240, italics added).
Adding Covariates – Controlling for Herd Reproduction
Another possibility would be to include the number of calves as a covariate in the analyses as this would provide a measure of how compensation and loss influence future herd size after statistically controlling for reproduction. Nevertheless, the number of calves (offspring t ) and predation compensation were strongly correlated (Table 7a), and including both in our analyses led to problems in relation to collinearity. High, or even moderate, collinearity is problematic when effects are weak (as in this study) as it may cause non-significant parameter estimates (i.e., the precision of the estimates decrease, see Licht 1995), compared to a situation without collinearity. With collinearity problems removed, variables may become significant, indicating that problems pertaining to collinearity may render significant terms non-significant (Zuur et al. 2010:9). More to the point, if collinearity is ignored it is possible to end up with a statistical analysis where nothing is significant, but where dropping one predictor may make others significant, or even change the sign of estimated parameters (Zuur et al. 2010:9). When fitting models with both number of calves and predation compensation included as covariates, the models were sensitive for which covariates and interactions that were included/excluded (results not shown). This indicates that the correlation between predation compensation and the number of calves caused collinearity problems, and we thus decided to exclude the number of calves from the analyses.Footnote 14
Second, as previously mentioned having a large herd gives reindeer husbandry units the possibility to report much larger losses than husbandry units with smaller herds. In other words, present herd size and loss (and compensation) is correlated (Table 7b). Consequently, since Næss and Bårdsen (2010) found that for Finnmark present herd size was a good predictor for future herd size, predation compensation can represent a proxy for herd size. In other words, the collinearity between present herd size and predation compensation can arguably be taken to indicate that it is present herd size and not predation compensation that is responsible for the results presented in this study. To further compound the problem, because of the collinearity between present herd size and predation compensation we could not include present herd size as a covariate and thus estimate the effect of predation compensation on future herd size after controlling for present herd size.
Thus, important factors such as present herd size and number of calves could not be estimated in the same model due to the degree of collinearity with predation compensation (Table 7a,b). In essence, we were left with a choice whether to include the predictors for which we had expectations (from a theoretical point of view) or to replace them with other possible important covariates. From a statistical point of view, such problems fall under the purview of specification error, and in this case it could be argued that the problem is mainly related to estimating a model with the wrong set of predictors. Nevertheless, decisions regarding which predictors to include or exclude cannot be assessed statistically but must be based on theoretical considerations relevant to the hypotheses tested (Berry and Feldman 1985; Licht 1995). Consequently, we chose to focus on predation compensation because this was the variable we had both a theoretical interest in and a priori expectations for (see Fox 1991:15 for a similar argument). Moreover, predation compensation represents a distinct theoretical concept measuring both reindeer loss and economic input.
Solving Collinearity Problems – Constructing a New Variable
Nevertheless, it could be argued that it is still important to account for some measure of reproduction. Consequently, we made a new variable measuring the number of calves produced per reindeer (offspring per reindeer t , which is a combination of both herd size and number of calves). A correlation test between this variable and predation compensation revealed that previous problems pertaining to collinearity was greatly reduced (Table 7c). Based on this finding we re-fitted the best models presented in the main text (Tables 1, 2 and 3) using the number of calves produced per reindeer as a covariate. These analyses revealed that while the number of calves per reindeer was a statistically significant positive predictor for future herd size, the presence of this predictor changed neither the original results nor the interpretation of them (i.e., the point estimates, the precision of the point estimates and the direction of the point estimates did not change dramatically (Tables 8, 9 and 10). This indicates that the results presented in the main text are not sensitive to underlying confounding with the aforementioned variables not included in the analyses.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Næss, M.W., Bårdsen, BJ., Pedersen, E. et al. Pastoral Herding Strategies and Governmental Management Objectives: Predation Compensation as a Risk Buffering Strategy in the Saami Reindeer Husbandry. Hum Ecol 39, 489–508 (2011). https://doi.org/10.1007/s10745-011-9398-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10745-011-9398-7