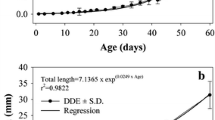
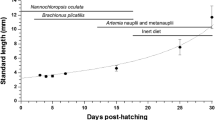
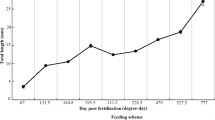
Abstract
Spotted sand bass Paralabrax maculatofasciatus is a potential aquaculture species in Northwest Mexico. In the last few years it has been possible to close its life cycle and to develop larviculture technology at on pilot scale using live food, however survival values are low (11%) and improvements in growth and survival requires the study of the morpho-physiological development during the initial ontogeny. In this research digestive activity of several enzymes were evaluated in larvae, from hatching to 30 days after hatching (dah), and in live prey (rotifers and Artemia), by use of biochemical and electrophoretic techniques. This paper, is the first of two parts, and covers only the biochemical analysis. All digestive enzyme activities were detected from mouth opening; however the, maximum activities varied among different digestive enzymes. For alkaline protease and trypsin the maximum activities were detected from 12 to 18 dah. Acid protease activity was observed from day 12 onwards. The other digestive enzymes appear between days 4 and 18 after hatching, with marked fluctuations. These activities indicate the beginning of the juvenile stage and the maturation of the digestive system, in agreement with changes that occur during morpho-physiological development and food changes from rotifers to Artemia. All enzymatic activities were detected in rotifers and Artemia, and their contribution to enhancement the digestion capacity of the larvae appears to be low, but cannot be minimised. We concluded that the enzymatic equipment of P. maculatofasciatus larvae is similar to that of other marine fish species, that it becomes complete between days 12 and 18 after hatching, and that it is totally efficient up to 25 dah.



Similar content being viewed by others
References
Alvarez-González CA, Ortíz-Galindo JL, Dumas S, Martínez-Díaz SF, Hernández-Ceballos DE, Grayeb del Alamo T, Moreno-Legorreta M, Peña-Martínez R, Civera-Cerecedo R (2001) Effect of stocking density on the growth and survival of spotted sand bass Paralabrax maculatofasciatus larvae in a closed recirculating system. J World Aquacult Soc 32:130–137
Alvarez-González CA, Cervantes-Trujano M, Tovar-Ramírez D, Conklin DE, Nolasco H, Gisbert E, Piedrahita R (2005) Development of digestive enzymes in California halibut Paralichthys californicus larvae. Fish Physiol Biochem 31:83–93
Anguas-Vélez BH, Civera-Cerecedo R, Contreras-Olguín M, Rueda-Jasso RA, Guillaume J (2000) Preliminary study on the timing of weaning of spotted sand bass (Paralabrax maculatofasciatus) larvae with a prepared diet: effects on growth and survival. J Appl Aquacult 10:1–15
Anson ML (1938) The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J Gen Physiol 22:79–89
Ásgeirsson B, Bjarnasson JB (1991) Structural and kinetic properties of chymotrypsin from Atlantic cod (Gadus morhua). Comparison with bovine chymotrypsin. Comp Biochem Physiol 99B:327–335
Avilés-Quevedo A, McGregor-Pardo U, Rodríguez-Ramos R, Hirales-Cosio O, Huerta-Bello M, Izawa M (1995) Biología y cultivo de la cabrilla arenera Paralabrax maculatofasciatus (Steindachner, 1869). SEPESCA, México
Baglole CJ, Goff GP, Wright GM (1998) Distribution and ontogeny of digestive enzymes in larval yellowtail and winter flounder. J Fish Biol 53:767–784
Bergmeyer HV (1974) Phosphatases. Methods of enzymatic analysis, vol 2. Academic Press
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Buchet V, Zambonino-Infante JL, Cahu CL (2000) Effect of lipid level in a compound diet on the development of red drum (Sciaenops ocellatus) larvae. Aquaculture 184:339–347
Cahu CL, Zambonino-Infante JL (1994) Early weaning of sea bass (Dicentrarchus labrax) larvae with a compound diet: effect on digestive enzymes. Comp Biochem Physiol 109A:213–222
Cahu CL, Zambonino-Infante JL (1997) Is the digestive capacity of marine fish larvae sufficient for compound diet feeding? Aquacult Int 5:151–160
Cara JB, Moyano FJ, Cárdenas S, Fernández-Díaz C, Yúfera M (2003) Assessment of digestive enzyme activities during larval development of white bream. J Fish Biol 63:48–58
Chen BN, Qin JG, Kumar MS, Hutchinson W, Clarke S (2006) Ontogenetic development of the digestive system in yellowtail kingfish Seriola lalandi larvae. Aquaculture 256:489–501
Clark J, Murray KR, Stark JR (1986) Protease development in dover sole (Solea solea (L.)). Aquaculture 53:253–262
Copeland RA (1996) Structural components of enzymes. In: Enzymes, a practical introduction to structure, mechanism and data analysis. Wiley, New York, pp 35–65
Cousin JCB, Baudin-Laurencin F, Gabaudan J (1987) Ontogeny of enzymatic activities in fed and fasting turbot, Scophthalmus maximus L. J Fish Biol 30:15–33
Cuvier-Péres A, Kestemont P (2002) Development of some digestive enzymes in Eurasian perch larvae Perca fluviatilis. Fish Physiol Biochem 24:279–285
Díaz M, Moyano FJ, García-Carreño LF, Alarcón FJ, Sarasquete MC (1997) Substrate-SDS-PAGE determination of protease activity through larval development in sea bream. Aquacult Inter 5:461–471
Diaz JP, Mani-Ponset L, Blasco C, Connes R (2002) Cytological detection of the main phases of lipid metabolism during early post-embryonic development in three teleost species Dicentrarchus labrax, Sparus aurata and Stizostedion lucioperca. Aquat Living Resour 15:196–178
Erlanger B, Kokowsky N, Cohen W (1961) The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95:271–278
Garía-Ortega A, Verreth JAJ, Coutteau P, Segner H, Huisman EA, Sorgeloos P (1998) Biochemical and enzymatic characterization of decapsulated cysts and nauplii of the brine shrimp Artemia at different developmental stages. Aquaculture 161:501–514
Gawlicka A, Parent B, Horn MH, Ross N, Opstad I, Torrinsen OJ (2000) Activity of digestive enzymes in yolk-sac larvae of Atlantic halibut (Hippoglossus hippoglossus): indication of readiness for first feeding. Aquaculture 184:303–314
German DP, Horn MH, Gawlicka A (2004) Digestive enzyme activities in herbivorous and carnivorous prickleback fishes (Teleostei: Stichaeidae): ontogenetic, dietary, and phylogenetic effects. Physiolog Biochem Zool 77:789–804
Hakim Y, Rowland SJ, Guy JA, Mifsud C, Uni Z, Harpaz S (2007) Effects of genetic strain and holding facility on the characteristics of alkaline phosphatase and brush border enzymes in silver perch (Bidyanus bidyanus). Aquacul Res 38:361–372
Hoehne-Reitan K, Kjorsvik E, Reitan KI (2001) Bile salt-dependent lipase in larval turbot, as influenced by density and lipid content of fed prey. J Fish Biol 58:746–754
Kunitz M (1947) Crystalline soybean trypsin inhibitor II. General properties. J Gen Physiol 30:291–310
Kurokawa T, Shiraishi M, Suzuki T (1998) Quantification of exogenous proteases derived from zooplankton in the intestine of Japanese sardine (Sardinops melanoticus) larvae. Aquaculture 161:491–499
Kvåle A, Mangor-Jensen A, Moren M, Espe M, Hamre K (2007) Development and characterisation of some intestinal enzymes in Atlantic cod (Gadus morhua L.) and Atlantic halibut (Hippoglossus hippoglossus L.) larvae. Aquaculture 264:457–468
Maraux S, Louvard D, Baratti J (1973) The aminopeptidase from hog-intestinal brush border. Biochem Biophys Acta 321:282–295
Martínez MI, Moyano FJ, Fernández-Díaz C, Yúfera M (1999) Digestive enzyme activity during larval development of the Senegal sole (Solea senegalensis). Fish Physiol Biochem 21:317–323
Morais S, Lacuisse M, Conceição LEC, Dinis MT, Rønnestad I (2004) Ontogeny of the digestive capacity of Senegalese sole (Solea senegalensis), with respect to digestion, absorption and metabolism of amino acids from Artemia. Mar Biol 145:243–250
Moyano FJ, Diaz M, Alarcón FJ, Sarasquete MC (1996) Characterization of digestive enzyme activity during larval development of gilthead seabream (Sparus aurata). Fish Physiol Biochem 15:121–130
Munilla-Morán R, Saborido-Rey F (1996) Digestive enzymes in marine species. II. Amylase activities in gut from seabream (Sparus aurata), turbot (Scophthalmus maximus) and red fish (Sebastes mentella). Comp Biochem Physiol 113B:827–834
Ozkizilcik S, Chu F-LE, Place AR (1996) Ontogenetic changes of lipolytic enzymes in striped bass (Morone saxatilis). Comp Biochem Physiol 113B:631–637
Pedersen BH, Andersen KP (1992) Induction of trypsinogen in herring larvae (Clupea harengus). Mar Biol 112:559–565
Pedersen BH, Uberchär B, Kurokawa T (2003) Digestive response and rates of growth in pre-leptocephalus larvae of the Japanese eel Anguilla japonica reared on artificial diets. Aquaculture 215:321–338
Peña-Martínez R, Dumas S, Villalejo-Fuerte M, Ortíz-Galindo JL (2003) Ontogenetic development of the digestive tract in reared spotted sand bass Paralabrax maculatofasciatus larvae. Aquaculture 219:633–644
Péres A, Cahu CL, Zambonino-Infante JL, Legall MM, Quazuguel P (1996) Amylase and trypsin responses to intake of dietary carbohydrate and protein depend on the developmental stage in sea bass (Dicentrarchus labrax) larvae. Fish Physiol Biochem 15:237–242
Perez-Casanova JC, Murray HM, Gallant JW, Ross NW, Douglas SE, Johnson SC (2006) Development of the digestive capacity in larvae of haddock (Melanogrammus aeglefinus) and Atlantic cod (Gadus morhua). Aquaculture 251:377–401
Ribeiro L, Zambonino-Infante JL, Cahu CL, Dinos MT (2002) Digestive enzymes profile of Solea senegalensis post larvae fed Artemia and a compound diet. Fish Physiol Biochem 27:61–69
Robyt JF, Whelan WJ (1968) The β-amylases. In: Radley JA (ed) Starch and its derivatives. Academic Press, London, pp 477–497
Sidell BD, Hazel JR (2002) Triacylglycerol lipase activities in tissues of Antarctic fishes. Polar Biol 1:517–522
Theilacker GH, Kimball AS (1984) Rotifers and copepods as larval fish foods. In: Hunter JR (ed) Synopsis of culture methods for marine fish larvae, CalCOFI Rep., vol XXV. pp 80–86
Versaw W, Cuppett SL, Winters DD, Williams LE (1989) An improved colorimetric assay for bacterial lipase in nonfat dry milk. J Food Sci 54:232–254
Versichelle D, Léger P, Lavens P, Sorgeloos P (1989) L’utilisation d’artémia. In: Barnabé G (ed) Aquaculture, Technique et Documentation, Lavoisier, Paris
Walter HE (1984) Proteinases: methods with hemoglobin, casein and azocoll as substrates. In: Bergmeyer HJ (ed) Methods of enzymatic analysis, vol V. Verlag Chemie, Weinham
Zambonino-Infante JL, Cahu CL (1994) Development and response to a diet change of some digestive enzymes in sea bass (Dicentrarchus labrax) larvae. Fish Physiol Biochem 12:399–408
Zambonino-Infante JL, Cahu CL (1999) High dietary lipid levels enhance digestive tract maturation and improve Dicentrarchus labrax larval development. J Nutr 129:1195–1200
Zambonino-Infante JL, Cahu CL (2001) Ontogeny of the gastrointestinal tract of marine fish larvae. Comp Biochem Physiol 130C:477–487
Zambonino-Infante JL, Cahu CL (2007) Dietary modulation of some digestive enzymes and metabolic processes in developing marine fish: applications to diet formulation. Aquaculture 268:98–105
Acknowledgements
This research was supported by the institutional projects: CGPI 998022, 20010825 and 20020357, by the Consejo Nacional de Ciencia y Tecnología, Mexico (CONACyT) Project 31666B, by the Universidad de Almería, and by the Centro de Investigaciones Biológicas del Noroeste (CIBNOR). We also want to thank the Comisión de Operación y Fomento de Actividades Académicas (COFAA-IPN) and the Programa Institucional para la Formación de Investigadores (PIFI-IPN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alvarez-González, C.A., Moyano-López, F.J., Civera-Cerecedo, R. et al. Development of digestive enzyme activity in larvae of spotted sand bass Paralabrax maculatofasciatus. 1. Biochemical analysis. Fish Physiol Biochem 34, 373–384 (2008). https://doi.org/10.1007/s10695-007-9197-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-007-9197-7