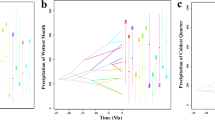
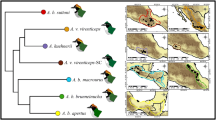
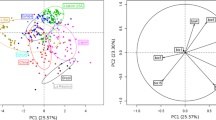
Abstract
Niche conservatism (NC) presence is a controversial question in evolutionary ecology. In Drosophila, little is known about which is the preponderant evolutionary pattern, since the adaptive radiation hypothesis first proposed by Throckmorton assumed niche divergence (ND) according to a niche occupancy scenario. Nevertheless, this hypothesis has not yet been straightforwardly tested. Our aim here was to test the role of NC patterns across evolution of American drosophilids belonging to the tripunctata and virilis-repleta lineages of the Drosophila subgenus, through measures of geographical, abiotic and biotic niche overlap and evaluations regarding the presence of phylogenetic signal or niche identity. We recovered phylogenetic signal attributable to phylogenetic niche conservatism when all species were analyzed together, but not in more restricted groups. Identity tests showed that niche equivalency was seldom rejected for the tripunctata lineage species. So, in general, neither the results for the Drosophila subgenus nor those for the tripunctata lineage support the hypothesis of an adaptive radiation. Notwithstanding, there were also several isolated cases supporting a scenario of ND, and ecological speciation was evident in some of the evaluated sister species pairs.



Similar content being viewed by others
References
Bächli G (2012) TaxoDros: the database on taxonomy of Drosophilidae, v. 1.03, Database 2011/12. http://www.taxodros.uzh.ch/
Bächli G (2015) TaxoDros: the database on taxonomy of Drosophilidae, v. 1.04, Database 2015/3. http://www.taxodros.uzh.ch/
Bächli G, Vilela CR, Ratcov V (2000) Morphological differences among Drosophila paraguayensis Duda 1927 and its close relatives (Diptera, Drosophilidae). Mitt Schweiz Entomol Ges 73:67–92
Blauth ML, Gottschalk MS (2007) A novel record of Drosophilidae in the Cerrado biome of the Mato Grosso, west-central Brazil. Drosoph Inf Serv 90:90–96
Blomberg SP, Garland T Jr, Ives AR (2003) Testing for phylogenetic signal in comparative data: behavior traits are more labile. Evolution 57(4):717–745
Chaves NB, Tidon R (2008) Biogeographical aspects of drosophilids (Diptera, Drosophilidae) of the Brazilian savanna. Rev Bras Entomol 52(3):340–348
Cooper N, Jetz W, Freckleton RP (2010) Phylogenetic comparative approaches for studying niche conservatism. J Evol Biol 23(12):2529–2539
Coyne JA, Orr HA (2004) Speciation. Sinauer Associates, Sunderland
Crisp MD, Cook LG (2012) Phylogenetic niche conservatism: What are the underlying evolutionary and ecological causes? New Phytol 196:681–694
De Ré FC (2011) Filogeografia de populações Brasileiras de Drosophila maculifrons e Drosophila griseolineata. Dissertation, Universidade Federal de Santa Maria
De Toni DC, Hofmann PRP, Valente VLS (2001) First record of Zaprionus indianus (Diptera, Drosophilidae) in the state of Santa Catarina, Brazil. Biotemas 14(1):71–85
Durando CM, Baker RH, Etges WJ et al (2000) Phylogenetic analysis of the repleta species group of the genus Drosophila using multiple sources of characters. Mol Phylogenet Evol 16:296–307
Elith J, Leathwick JR (2009) Species distribution models: ecological explanation and prediction across space and time. Annu Rev Ecol Evol Syst 40:677–697
Freckleton RP, Harvey PH, Pagel M (2002) Phylogenetic analysis and comparative data: a test and review of evidence. Am Nat 160(6):712–726
Freckleton RP, Jetz W (2009) Space versus phylogeny: disentangling phylogenetic and spatial signals in comparative data. Proc R Soc B 276:21–30
Frota-Pessoa O (1952) Flower-feeding Drosophilidae. Drosoph Inf Serv 26:101–102
Glennon KL, Rissler LJ, Church SA (2012) Ecogeographic isolation: a reproductive barrier between species and between cytotypes in Houstonia (Rubiaceae). Evol Ecol 26:909–926
Godoy-Herrera R, Burnet B, Connolly K (2005) Hybrid disadvantage in the larval foraging behaviour of the two Neotropical species of Drosophila pavani and Drosophila gaucha. Genetica 124:33–40
Godsoe W (2010) Regional variation exaggerates ecological divergence in niche models. Syst Biol 59(3):298–306
Gotelli NJ, Graves GR (1996) Null models in ecology. Smithsonian Institution Press, Washington
Gotelli NJ, Entsminger GL (2001) EcoSim: null models software for ecology. Version 7.0. Acquired Intelligence Inc. and Kesey-Bear. http://www.uvm.edu/~ngotelli/EcoSim/EcoSim.html
Gottschalk MS (2008) Utilização de recursos tróficos por espécies Neotropicais de Drosophilidae (Diptera). D. Phil Thesis, Universidade Federal do Rio Grande do Sul
Gottschalk MS, Bizzo L, Döge JS et al (2009) Drosophilidae (Diptera) associated to fungi: differential use of resources in anthropic and Atlantic Rain Forest areas. Iheringia Ser Zool 99(4):442–448
Graham CH, Ron SR, Santos JC et al (2004) Integrating phylogenetics and environmental niche models to explore speciation mechanisms in dendrobatid frogs. Evolution 58(8):1781–1793
Grimaldi DA (1990) A phylogenetic, revised classification of the genera in the Drosophilidae (Diptera). Bull Am Mus Nat Hist 197:1–13
Hardy OJ, Pavoine S (2012) Assessing phylogenetic signal with measurement error: A comparison of Mantel tests, Blomberg et al’.s K, and phylogenetic distograms. Evolution 66(8):2614–2621
Harmon LJ, Glor GE (2010) Poor statistical performance of the Mantel test in phylogenetic comparative analyses. Evolution 64(7):2173–2178
Hatadani LM, McInerney JO, de Medeiros HF et al (2009) Molecular phylogeny of the Drosophila tripunctata and closely related species groups (Diptera: Drosophilidae). Mol Phylogenet Evol 51:595–600
Heed WB (1956) Apuntes sobre la ecologia y la dispersion de los Drosophilidae (Diptera) de El Salvador. Comunicaciones del Instituto Tropical de Investigaciones Cientificas El Salvador San Salvador 5:59–74
Hernandez PA, Graham CH, Master LL et al (2006) The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 29:773–785
Hijmans RJ, Cameron SE, Parra JL (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Kamino LHY, Stehmann JR, Amaral S et al (2012) Challenges and perspectives for species distribution modelling in the neotropics. Biol Lett 8:324–326
Kellerman V, van Heerwaarden B, Sgrò CM et al (2009) Fundamental evolutionary limits in ecological traits drive Drosophila species distributions. Science 325:1244–1246
Kellerman V, Overgaard J, Hoffmann AA et al (2012) Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. PNAS 109(40):16228–16233
Kellermann V, LoeschckeV HoffmannAA et al (2012) Phylogenetic constraints in key functional traits behind species’ climate niches: patterns of desiccation and cold resistance across 95 Drosophila species. Evolution 66:3377–3389
Kozak KH, Wiens JJ (2006) Does niche conservatism promotes speciation: a case study in north American salamanders. Evolution 60(12):2604–2621
Kozak KH, Wiens JJ (2010) Niche conservatism drives elevational diversity patterns in appalachian salamanders. Am Nat 176(1):40–54
Lobo JM, Tognelli MF (2011) Exploring the effects of quantity and location of pseudo-absences and sampling biases on the performance of distribution models with limited point occurrence data. J Nat Conserv 19:1–7
Losos JB (2008) Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol Lett 11:995–1007
Maddison WP, Maddison DR (2011) Mesquite: a modular system for evolutionary analysis. Version 2.75. http://mesquiteproject.org
Markow TA, O’Grady P (2008) Reproductive ecology of Drosophila. Funct Ecol 22:747–759
Martins MB, Santos RCO (2007) Sítios de criação de Drosophila na Reserva Mocambo, Belém. Pará. In: Mocambo: Diversidade e Dinâmica biológica da Área de Pesquisa Ecológica do Guampa (APEG). Belém, Museu P. Emilio Goeldi/Embrapa Amazonia oriental, pp 318–331
Mcnyset M (2009) Ecological niche conservatism in North American freshwater fishes. Biol J Linn Soc Lond 96(2):282–295
Medina-Muñoz MC, Godoy-Herrera R (2004) Dispersal and prepupation behavior of Chilean sympatric Drosophila species that breed in the same site in nature. Behav Ecol 15(2):316–322
Morales-Hojas R, Vieira J (2012) Phylogenetic patterns of geographical and ecological diversification in the subgenus Drosophila. PLoS One 7(11):e49552
Mota NR, Robe LJ, Valente VLS et al (2008) Phylogeny of the Drosophila mesophragmatica group (Diptera, Drosophilidae): an example of Andean evolution. Zool Sci 25(5):526–532
Münkemüller T, Lavergne S, Bzeznik B et al (2012) How to measure and test phylogenetic signal. Methods Ecol Evol 3:743–756
O’Grady PM, Markow TA (2009) Phylogenetic taxonomy in Drosophila: problems and prospects. Fly 3(1):10–14
Oliveira DCSG, Almeida FC, O’Grady PM (2012) Monophyly, divergence times, and evolution of host plant use inferred from a revised phylogeny of the Drosophila repleta species group. Mol Phylogenet Evol 64:533–544
Pagel M (1999) Inferring the historical patterns of biological evolution. Nature 401:877–884
Pearman PB, Guisan A, Broennimann O et al (2008) Niche dynamics in space and time. Trends Ecol Evol (Amst) 23(3):149–158
Peterson AT, Soberón J, Sánchez-Cordero V (1999) Conservatism of ecological niches in evolutionary time. Science 285:1265–1267
Peterson AT (2011) Ecological niche conservatism: a time-structured review of evidence. J Biogeogr 38:817–827
Philippe H, Brinkmann H, Lavrov VD et al (2011) Resolving difficult phylogenetic questions: why more sequences are not enough. PLoS Biol 9(3):1–10
Phillips JS, Dudík M, Schapire RE (2004) A maximum entropy approach to species distribution modeling. In: Proceedings of the twenty-first international conference on machine learning. AMC Press, New York, pp 655–662
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259
Phillips SJ, Dudik M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31:161–175
Pipkin SB, Rodriguez RL, León J (1966) Plant host specificity among flower-feeding Neotropical Drosophila (Diptera: Drosophilidae). Am Nat 100:135–156
Pyron A, Burbrink FT (2009) Lineage diversification in a widespread species: roles for niche divergence and conservatism in the common kingsnake, Lampropeltis getula. Mol Ecol 18:3443–3457
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Revell LJ, Harmon LJ, Collar DC (2008) Phylogenetic signal, evolutionary process and rate. Syst Biol 57(4):591–601
Revell LJ (2012) Phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3:217–223
Robe LJ, Valente VLS, Budnik M et al (2005) Molecular phylogeny of the subgenus Drosophila (Diptera, Drosophilidae) with an emphasis on Neotropical species and groups: a nuclear versus mitochondrial gene approach. Mol Phylogenet Evol 36:623–640
Robe LJ, Loreto ELS, Valente VLS (2010a) Radiation of the, Drosophila“subgenus (Drosophilidae, Diptera) in the Neotropics. J Zool Syst Evol Res 48(4):310–321
Robe LJ, Valente VLS, Loreto ELS (2010b) Phylogenetic relationships and macro-evolutionary patterns within the Drosophila tripunctata “radiation” (Diptera: Drosophilidae). Genetica 138:725–735
Robe LJ, de Ré FC, Ludwig A et al (2013) The Drosophila flavopilosa species group (Diptera, Drosophilidae): an array of exciting questions. Fly 7(2):59–69
Rocchini D, Hortal J, Lengyel S (2011) Accouting for uncertainty when mapping species distribution: the need for map of ignorance. Prog Phys Geogr 35(2):211–226
Roque F, Tidon R (2008) Eight new records of drosophilids (Insecta; Diptera) in the Brazilian savanna. Drosoph Inf Serv 91:94–98
Rosenberg MS, Anderson CD (2011) PASSaGE: pattern analysis, spatial statistics and geographic exegesis. Methods Ecol Evol 2(3):229–232
Rundle HD, Nosil P (2005) Ecological speciation. Ecol Lett 8:336–352
Russo CM, Mello B, Frazão A et al (2013) Phylogenetic analysis and a time tree for a large drosophilid data set (Diptera: Drosophilidae). Zool J Linn Soc 169:765–775
Saavedra CCR, Callegari-Jaques SM, Napp M et al (1995) A descriptive and analytical study of four Neotropical drosophilid communities. J Zool Syst Evol Res 33:62–74
Saavedera CCR, Valente VLS, Napp M (1995) An ecological/genetic approach to the study of enzymatic polymorphisms in Drosophila maculifrons. Rev Bras Genet 18(2):147–164
Salgado-Laboriau ML (1994) O Período Quaternário. História Ecológica da Terra. Editora Edgard Blücher, São Paulo, pp 255–280
Santos RCO, Vilela CR (2005) Breeding sites of Neotropical Drosophilidae (Diptera). IV. Living and fallen flowers of Sessea brasiliensis and Cestrum spp. (Solanaceae). Rev Bras Entomol 49(4):544–551
Stockwell RB, Peterson AT (2002) Effects of sample size on accuracy of species distribution models. Ecol Model 148(1):1–13
Tamura K, Subramanian S, Kumar S (2004) Temporal patterns of fruit fly (Drosophila) evolution revealed by mutation clocks. Mol Biol Evol 21:36–44
Tatarenkov A, Ayala FJ (2001) Phylogenetic relationships among species groups of the virilis-repleta radiation of Drosophila. Mol Phylogenet Evol 21(2):327–331
Throckmorton LH (1975) The phylogeny, ecology and geography of Drosophila. In: King RC (ed) Handbook of genetics. Plenum, New York, pp 421–469
Tosi D, Martins M, Vilela CR et al (1990) On a new cave-dwelling species of bat-guano-breeding Drosophila closely related to D. repleta Wollaston (Diptera, Drosophilidae). Rev Bras Genet 13(1):19–31
Val FC, Marques MD, Vilela CR (1981) Drosophilidae of Neotropical region. In: Ashburner M, Carson HL, Thompson JN (eds) The genetics and biology of Drosophila. Academic Press, Orlando, pp 123–168
Valadão H, Hay JDV, Tidon R (2010) Temporal dynamics and resource availability for drosophilid fruit flies (Insecta, Diptera) in a gallery forest in the Brazilian savanna. Int J Ecol 1:1–7
van der Linde KVD, Houle D (2008) A supertree analysis and literature review of the genus Drosophila and closely related genera (Diptera, Drosophilidae). Insect Syst Evol 39:241–267
van der Linde KVD, Houle D, Spicer GS et al (2010) A supermatrix-based molecular phylogeny of the family Drosophilidae. Genet Res (Camb) 92:25–38
Wang IJ, Glor RE, Losos JB (2013) Quantifying the roles of ecology and geography in spatial genetic divergence. Ecol Lett 16:175–182
Warren DL, Glor RE, Turelli M (2008) Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62(11):2868–2883
Warren DL, Glor RE, Turelli M (2010) ENMTools: a toolbox for comparative studies of environmental niche models. Ecography 33:607–611
Wiens JJ (2004) Speciation and ecology revisited: phylogenetic niche conservatism and the origin of species. Evolution 58(1):193–197
Wiens JJ, Graham CH (2005) Niche conservatism: integrating evolution, ecology, and conservation biology. Annu Rev Ecol Evol Syst 36:519–539
Wiens JJ, Ackerly DD, Allen AP et al (2010) Niche conservatism as an emerging principle in ecology and conservation biology. Ecol Lett 13:1310–1324
Wisz MS, Hijmans RJ, Peterson AT et al (2008) Effects of sample size on the performance of species distribution models. Divers Distrib 14:763–773
Yassin A (2013) Phylogenetic classification of the Drosophilidae Rondani (Diptera): the role of morphology in the postgenomic era. Syst Entomol 38:349–364
Yoder JB, Clancey E, Roches SD et al (2010) Ecological opportunity and the origin of adaptive radiations. J Evol Biol 23:1581–1596
Yotoko KSC, Medeiros HF, Solferini VN et al (2003) A molecular study of the systematics of the Drosophila tripunctata group and the tripunctata radiation. Mol Phylogenet Evol 28:614–619
Acknowledgments
We are grateful to Dr. Daniel A. S. Graichen, Dr. Elgion L. S. Loreto, Dr. Fernando F. Franco, Dr. Francisco C. C. Barreto, Dr. Hermes J. Schmitz, Dra. Juliana Cordeiro and Dra. Vera L. S. Valente for their contributions to the manuscript. We also are grateful to the Brazilian Funding Agency Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support (Universal-CNPq 14/2013, Process Number 471174/2013-0).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1.
(A) Phylogenetic relationships presented by 24 of the 25 evaluated species, as modified from the total evidence tree (TET) provided for the Drosophila subgenus by Robe et al (2010a), with clades 12, 22, 32 and 61 enclosed within doted or square checkered rectangles for the tripunctata or virilis-repleta lineage species, respectively. Drosophila roehrae was used in the identity tests but not in the phylogenetic signal analyses, since it was not included in the TET of Robe et al (2010a) – although the nuclear marker amd positioned this species within clade 32. (B) Breeding sites reconstruction based on a compilation of presence/absence data performed for 20 of the Drosophila subgenus species (see references on the main text). Only autapomorphies were included (for a complementation see Fig. 1b within the manuscript). Black squares represent recorded emergence from the resource. Each character presented Consistence (CI) and Retention (RI) indexes of 1.0 and 0.0, respectively. (PNG 1305 kb)
Figure S2.
Interactive map of sampling sites per species [to be opened in Quantum GIS (http://www.qgis.org/en/site/)] (RAR 7256 kb)
Figure S3.
Relative frequencies distribution of pairwise comparisons regarding range overlap, abiotic (measured through Hellinger's I and Schoener’s D statistics) and biotic (measured through Czekanowski’s index) niche overlap values. Vertical bars represent the relative frequency of each overlap value among the set of pairwise comparisons. Arrows depict the values obtained for each of the evaluated sister species pair, (A) D. maculifrons/D. griseolineata, (B) D. mediopunctata/D. unipunctata, (C) D. cuaso/D. paraguayensis, (D) D. bandeirantorum/D. pallidipennis, (E) D. gaucha/D. pavani. (PNG 1150 kb)
Figure S4.
Niche equivalency hypothesis tests for each pair of sister species. Vertical bars represent the null hypothesis distribution of Schoener’s D (blue) and Hellinger’s I (green) statistics, obtained after pooling together the registers of two species and then randomizing them 200 times in order to produce two new samples with the same number of observations as the empirical data. Arrows show empirical D (blue) and I (green) values calculated for each sister species pair. Hypothesis of niche equivalency was rejected if empirical values were significantly lower than expected by chance. (PNG 1029 kb)
Supplementary spreadsheet 1.
Occurrence points used to generate the ENMs for each of the 25 studied species. The total number of points is different from that presented on Table 1 since some registers were grouped in the analysis due to the grid dimensions. (XLSX 98 kb)
Rights and permissions
About this article
Cite this article
Machado, S., Gottschalk, M.S. & Robe, L.J. Historical patterns of niche dynamics in Neotropical species of the Drosophila subgenus (Drosophilidae, Diptera). Evol Ecol 30, 47–67 (2016). https://doi.org/10.1007/s10682-015-9805-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-015-9805-4