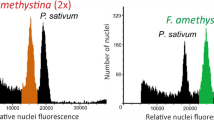
Abstract
‘Ecogeographic isolation’ describes the combined role of ecology and geography as a reproductive barrier, and an important component in speciation. Evidence increasingly shows that this form of isolation is important for maintaining the genetic integrity of populations and species. Further, ecogeographic isolation can be a reproductive barrier between polyploid individuals and their diploid progenitors. New ecoinformatic methods, which includes niche modeling and associated statistical assessments of these models with spatially explicit environmental data, allow us to test if ecogeographic isolation is a contributing isolating barrier between species and between cytotypes within a species. We tested the hypothesis that ecogeographic isolation contributes to isolation of species and cytotypes within species of the plant genus Houstonia. We found that species in this group occupied significantly different niches, which suggests ecogeographic isolation is a contributing reproductive barrier. We also found that diploid and tetraploid forms of H. longifolia show some level of ecogeographic isolation, but H. purpurea diploids and tetraploids did not. Our results suggest that ecogeographic isolation plays a role in reproductive isolation between Houstonia species and between cytotypes of H. longifolia.




Similar content being viewed by others
References
Adams KL, Wendel JF (2005) Novel patterns of gene expression in polyploid plants. Trends Genet 21:539–543
Baack EJ (2004) Cytotype segregation on regional and microgeographic scales in snow buttercups (Ranunculus adoneus: Ranunculaceae). Am J Bot 91:1783–1788
Baack EJ (2005) Ecological factors influencing tetraploid establishment in snow buttercups (Ranunculus adoneus, Ranunculaceae): minority cytotype exclusion and barriers to triploid formation. Am J Bot 92:1827–1835
Baack EJ, Stanton ML (2005) Ecological factors influencing tetraploid speciation in snow buttercups (Ranunculus adoneus): Niche differentiation and tetraploid establishment. Evolution 59:1936–1944
Bradshaw HD Jr, Schemske DW (2003) Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature 426:176–178
Brochmann C, Brysting AK, Alsos IG, Borgen L, Grundt HH, Scheen AC, Elven R (2004) Polyploidy in arctic plants. Biol J Linn Soc 82:521–536
Buggs RJA, Pannell JR (2007) Ecological differentiation and diploid superiority across a moving ploidy contact zone. Evolution 61:125–140
Church SA (2003) Molecular phylogenetics of Houstonia (Rubiaceae): descending aneuploidy and breeding system evolution in the radiation of the lineage across North America. Mol Phylogenet Evol 27:223–238
Coyne J, Orr HA (2004) Speciation. Sinauer Associates Inc, Sunderland
Darwin C (1859) Origin of species, 6th edn. John Murray, London
Dobzhansky T (1937) Genetics and the origin of species. Columbia University Press, New York, NY
Elith J, Graham CH et al (2006) Novel methods improve prediction of species' distributions from occurrence data. Ecography 29:129–151
Felber F (1991) Establishment of a tetraploid cytotype in a diploid population: effect of relative fitness of the cytotypes. J Evolution Biol 4:195–207
Felber-Girard M, Felber F, Buttler A (1996) Habitat differentiation in a narrow hybrid zone between diploid and tetraploid Anthoxanthum alpinum. New Phytol 133:531
Fowler NL, Levin DA (1984) Ecological constraints on the establishment of a novel polyploid in competition with its diploid progenitor. Am Nat 124:703–711
Funk DJ, Nosil P, Etges WJ (2006) Ecological divergence exhibits consistently positive associations with reproductive isolation across disparate taxa. Proc Natl Acad Sci USA 103:3209–3213
Glennon K, Donaldson J, Church SA (2011) Evidence for hybridization between the endangered Roan Mountain bluet, Houstonia purpurea var. montana (Rubiaceae) and its common congener. The Journal of the Torrey Botanical Society 138(3):272–286
Graham CH, Ron SR, Santos JC, Schneider CJ, Moritz C (2004) Intergrating phylogenetics and environmetnal niche models to explore speciation mechanisms in Dendrobatid frogs. Evolution 58:1781–1793
Grant V (1981) Plant speciation. Columbia University Press, New York
Hernandez P, Graham C, Master L, Albert D (2006) The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 29:773–785
Hijmans RJ, Guarino L, Cruz M, Rojas E (2001) Computer tools for spatial analysis of plant genetic resources data: 1. DIVA-GIS. Plant Genet Resour Newslett 127:15–19
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25:1965–1978
Hijmans RJ, Gavrilenko T, Stephenson S, Bamberg J, Salas A, Spooner DM (2007) Geographical and environmental range expansion through polyploidy in wild potatoes (Solanum section Petota). Global Ecol Biogeogr 16:485–495
Husband BC, Sabara HA (2003) Reproductive isolation between autoetraploids and their diploid progenitors in fireweed Chamerion angustifolium (Onagracaea). New Phytol 161:703–713
Jakob S, Ihlow A, Blattner FR (2007) Combined ecological niche modelling and molecular phylogeography revealed the evolutionary history of Hordeum marinum (Poaceae)—niche differentiation, loss of genetic diversity, and speciation in Mediterranean Quaternary refugia. Mol Ecol 16:1727–1731
Johnson MTJ, Husband BC, Burton TL (2003) Habitat differentiation between diploid and tetraploid Galax urceolata (Diapensiaceae). Int J Plant Sci 164:703–710
Kay KM (2006) Reproductive isolation between two closely related hummingbird-pollinated neotropical gingers. Evolution 60:538–552
Kozak KH, Wiens JJ (2006) Does niche conservatism promote speciation? A case study in North American salamanders. Evolution 60:2604–2621
Kozak KH, Graham CH, Wiens JJ (2008) Integrating GIS-based environmental data into evolutionary biology. Trends Ecol Evol 23:141
Levin DA (1975) Minority cytotype exclusion in local plant populations. Taxon 24:35–43
Levin DA (1983) Polyploidy and novelty in flowering plants. Am Nat 122:1–25
Levin DA (2002) The role of chromosomal change in plant evolution. Oxford series in ecology and evolution. Oxford University Press, New York
Levin DA (2004) The ecological transition in speciation. New Phytol 161:91–96
Lewis W, Terrell EE (1962) Chromosomal races in eastern North American species of Hedyotis (Houstonia). Rhodora 64:313–323
Lowry DB, Modliszewski JL, Wright KM, Wu CA, Willis JH (2008) The strength and genetic basis of reproductive isolating barriers in flowering plants. Phil Trans R Soc Lond B 363:3009–3021
Lumaret R, Guillerm JL, Delay J, Loutfi AAL, Izco J, Jay M (1987) Polyploidy and habitat differentiation in Dactylis glomerata L. from Galicia (Spain). Oecologia 73:436–446
Lynch M, Force AG (2000) The origin of interspecific genomic incompatibility via gene duplication. Am Nat 156:590–605
Martin SL, Husband BC (2009) Influence of phylogeny and ploidy on species ranges of North American angiosperms. J Ecol 97:913–922
Masterson J (1994) Stomatal size in fossil plants—evidence for polyploidy in majority of angiosperms. Science 264:421–424
McCormack JE, Zellmer AJ, Knowles LL (2010) Does niche divergence accompany allopatric divergence in Aphelocoma Jays as predicted under ecological speciation? insights from tests with niche models. Evolution 64:1231–1244
Muller HJ (1942) Isolating mechanisms, evolution and temperature. Biol Symp 6:71–125
Pearson RG et al (2007) Predicting species’ distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J Biogeogr 34:102–117
Peterson AT, Pepas M, Soberón J (2008) Rethinking receiving under the operating characteristic analysis applications in ecological niche modeling. Ecol Model 213:63–72
Phillips SJ, Dudik M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31:161–175
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecol Model 190:231
Raabova J, Fischer M, Munzbergova Z (2008) Niche differentiation between diploid and hexaploid Aster amellus. Oecologia 158:463–472
Ramsey J, Schemske DW (1998) Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu Rev Ecol Syst 29:467–501
Ramsey J, Bradshaw HD, Schemske DW (2003) Components of reproductive isolation between the monkeyflowers Mimulus lewisii and M. cardinalis (Phrymaceae). Evolution 57:1520–1534
Rissler LJ, Hijmans RJ, Graham CH, Moritz C, Wake DB (2006) Phylogeographic lineages and species comparisons in conservation analyses: a case study of California herpetofauna. Am Nat 167:655–666
Rodriguez DJ (1996) A model for the establishment of polyploidy in plants. Am Nat 147:33
Sampoux JP, Huyghe C (2009) Contribution of ploidy-level variation and adaptive trait diversity to the environmental distribution of taxa in the ‘fine-leaved fescue’ lineage (genus Festuca subg. Festuca). J Biogeogr 36:1978–1993
Segraves KA, Thompson JN (1999) Plant polyploidy and pollination: floral traits and insect visits to diploid and tetraploid Heuchera grossulariifolia. Evolution 53:1114–1127
Smith SD, Peralta IE (2002) Ecogeographic surveys as tools for analyzing potential reproductive isolating mechanisms: an example using Solanum juglandifolium Dunal, S. ochranthum Dunal, S. lycopersoicoides Dunal, and S. sitiens I. M. Johnston. Taxon 51:341
Sobel JM, Chen GF Watt LR, Schemske DW (2010) The biology of speciation. Evolution 64:295–315
Soberón J, Peterson AT (2005) Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodiversity Info 2:1–10
Spooner DM, Gavrilenko T, Jansky SH, Ovchinnikova A, Krylova E, Knapp S, Simon R (2010) Ecogeography of ploidy variation in cultivated potato (Solanum sect. Petota). Am J Bot 97(12):2049–2060
Stebbins GL (1942) The role of isolation in the differentiation of plant species. Biol Symp 6:217–233
Stuessy TF, Weiss-Schneeweiss H, Keil DJ (2004) Diploid and polyploid cytotype distribution in Melampodium cinereum and M. leucanthum (Asteraceae, Heliantheae). Am J Bot 91:889–898
Terrell E (1996) Revision of Houstonia (Rubiaceae-Hedyotideae). Syst Bot Monogr 48:1–118
Thompson JN, Merg KF (2008) Evolution of polyploidy and the diversification of plant-pollinator interactions. Ecology 89:2197–2206
Warren DL, Glor RE, Turelli M (2008) Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62:2868–2883
Warren DL, Glor RE, Turelli M (2010) ENMTools: a toolbox for comparative studies of environmental niche models. Ecography 33:607–611
Wiens JJ, Graham CH, Moen DS, Smith SA, Reeder TW (2006) Evolutionary and ecological causes of the latitudinal diversity gradient in Hylid frogs: treefrog trees unearth the roots of high tropical diversity. Am Nat 168:579–596
Acknowledgments
The authors would like to thank R. Olsen for his guidance for the flow cytometry analyses, D. Warren for his help with the ENMTools analyses, and K. Costantini for help with data collection. This manuscript benefited from insightful comments from anonymous reviewers and helpful discussions with J.J. Apodaca, J. Choiniere, J. Lill, M. Dudash, and G. Wimp. This research was funded by an NSF grant (DEB0816681) to SAC and a New England Botanical Society Graduate Research Award to KLG.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 1
Appendix 1
See Table 4.
Rights and permissions
About this article
Cite this article
Glennon, K.L., Rissler, L.J. & Church, S.A. Ecogeographic isolation: a reproductive barrier between species and between cytotypes in Houstonia (Rubiaceae). Evol Ecol 26, 909–926 (2012). https://doi.org/10.1007/s10682-011-9539-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-011-9539-x