Abstract
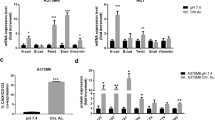
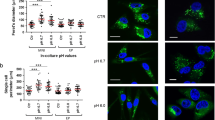
Tumor cell plasticity largely depends on epithelial-to-mesenchymal transition (EMT) and its reversion. It was ascertained that EMT characterizes disease progression, including melanoma malignancy. As most solid tumors, melanoma shows extracellular acidosis, we analyse the impact of acidic environment on the EMT development in human melanoma cells. Melanoma cells were exposed to an acidic extracellular environment (pH 6.7) and tested for EMT markers. We found that acidic cells express a significant up-regulation of mesenchymal markers (N-cadherin, Vimentin), transcription factors (Twist, NF-κB) and a significant, although modest, reduction of E-cadherin expression. Acidic cell also express an increased invasiveness through Matrigel associated with an up-regulation of MMP-9 activity. When we injected acidic cells intravenously into immunodeficient animals, we found a number of lung micrometastases not different from non-acidic cells. Indeed, they show a partial G1 cell cycle arrest, which might interfere with the growth of lung colonies. When we investigated the in vitro invasiveness and lung colonization of a mixed population of acidic and non acidic melanoma cells, we found that acidic cells promote in vitro invasiveness of non-acidic cells and this cooperation leads to an higher migration rate than acidic cells. Moreover, acidic cells cooperate for a better lung colonization of non-acidic cells, that represent the greater part of cells participating to lung micrometastases. We found evidence that acidity triggers in melanoma cells an EMT program, which although “incomplete”, potentiates migration rate and development of lung colonies into immunodeficient host of cells grown in standard pH.





Similar content being viewed by others
References
Alonso SR, Tracey L, Ortiz P, Pérez-Gómez B, Palacios J, Pollán M, Linares J, Serrano S, Sáez-Castillo AI, Sánchez L, Pajares R, Sánchez-Aguilera A, Artiga MJ, Piris MA, Rodríguez-Peralto JL (2007) A high-throughput study in melanoma identifies epithelial–mesenchymal transition as a major determinant of metastasis. Cancer Res 67:3450–3460
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial–mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119:1438–1449
Kalluri R, Weinberg RA (2009) The basics of epithelial–mesenchymal transition. J Clin Invest 119:1420–1428
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715
Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial–mesenchymal transitions. Nat Rev Mol Cell Biol 7:131–142
Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9:265–273
Scheel C, Weinberg RA (2012) Cancer stem cells and epithelial–mesenchymal transition: concepts and molecular links. Semin Cancer Biol 22:396–403
Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P (2010) Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial–mesenchymal transition and cancer stemness. Cancer Res 70:6945–6956
Thiery JP (2003) Epithelial–mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 15(6):740–746
Yang J, Weinberg RA (2008) Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14:818–829
Massague J (2008) TGF-β in cancer. Cell 134:215–230
Shi Y, Massagué J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113:685–700
Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ (2005) Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436:123–127
Gort EH, Groot AJ, van der Wall E, van Diest PJ, Vooijs MA (2008) Hypoxic regulation of metastasis via hypoxia-inducible factors. Curr Mol Med 8:60–67
Webb BA, Chimenti M, Jacobson MP, Barber DL (2011) Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 11:671–677
Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4:891–899
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Calorini L, Peppicelli S, Bianchini F (2012) Extracellular acidity as favouring factor of tumor progression and metastatic dissemination. Exp Oncol 34:79–84
Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, Mueller-Klieser W (2000) High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 60:916–921
Morita T, Nagaki T, Fukuda I, Okumura K (1992) Clastogenicity of low pH to various cultured mammalian cells. Mutat Res 268:297–305
Rottinger EM, Mendonca M (1982) Radioresistance secon-dary to low pH in human glial cells and Chinese hamster ovary cells. Int J Radiat Oncol Biol Phys 8:1309–1314
Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, Renner K, Timischl B, Mackensen A, Kunz-Schughart L, Andreesen R, Krause SW, Kreutz M (2007) Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109:3812–3819
Thews O, Gassner B, Kelleher DK, Schwerdt G, Gekle M (2006) Impact of extracellular acidity on the activity of P-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia 8:143–152
Chen TR (1977) In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res 104:255–262
Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49:6449–6465
Wike-Hooley JL, Haveman J, Reinhold HS (1984) The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol 2:343–366
Hsu MY, Wheelock MJ, Johnson KR, Herlyn M (1996) Shifts in cadherin profiles between human normal melanocytes and melanomas. J Investig Dermatol Symp Proc 1:188–194
Hazan RB, Phillips GR, Qiao RF, Norton L, Aaronson SA (2000) Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J Cell Biol 148:779–790
Krengel S, Grotelüschen F, Bartsch S, Tronnier M (2004) Cadherin expression pattern in melanocytic tumors more likely depends on the melanocyte environment than on tumor cell progression. J Cutan Pathol 31:1–7
De Wever O, Pauwels P, De Craene B, Sabbah M, Emami S, Redeuilh G, Gespach C, Bracke M, Berx G (2008) Molecular and pathological signatures of epithelial–mesenchymal transitions at the cancer invasion front. Histochem Cell Biol 130:481–494
Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, Chang SY, Lee OK, Wu KJ (2010) Bmi1 is essential in Twist1-induced epithelial–mesenchymal transition. Nat Cell Biol 12:982–992
Peppicelli S, Bianchini F, Contena C, Tombaccini D, Calorini L (2013) Acidic pH via NF-κB favours VEGF-C expression in human melanoma cells. Clin Exp Metastasis 30(8):957–967
Amiri KI, Richmond A (2005) Role of nuclear factor-kappa B in melanoma. Cancer Metastasis Rev 24:301–313
Kato Y, Lambert CA, Colige AC, Mineur P, Noël A, Frankenne F, Foidart JM, Baba M, Hata R, Miyazaki K, Tsukuda M (2005) Acidic extracellular pH induces matrix metalloproteinase-9 expression in mouse metastatic melanoma cells through the phospholipase D-mitogen-activated protein kinase signaling. J Biol Chem 280:10938–10944
Moellering RE, Black KC, Krishnamurty C, Baggett BK, Stafford P, Rain M, Gatenby RA, Gillies RJ (2008) Acid treatment of melanoma cells selects for invasive phenotypes. Clin Exp Metastasis 25:411–425
Friedl P, Wolf K (2003) Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer 3:362–374
Celià-Terrassa T, Meca-Cortés O, Mateo F, de Paz AM, Rubio N, Arnal-Estapé A, Ell BJ, Bermudo R, Díaz A, Guerra-Rebollo M, Lozano JJ, Estarás C, Ulloa C, Álvarez-Simón D, Milà J, Vilella R, Paciucci R, Martínez-Balbás M, de Herreros AG, Gomis RR, Kang Y, Blanco J, Fernández PL, Thomson TM (2012) Epithelial–mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest 122:1849–1868
Tsuji T, Ibaragi S, Hu GF (2009) Epithelial–mesenchymal transition and cell cooperativity in metastasis. Cancer Res 69:7135–7139
Rofstad EK, Mathiesen B, Kindem K, Galappathi K (2006) Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res 66:6699–6707
Jang A, Hill RP (1997) An examination of the effects of hypoxia, acidosis, and glucose starvation on the expression of metastasis-associated genes in murine tumor cells. Clin Exp Metastasis 15:469–483
Casey JR, Grinstein S, Orlowski J (2010) Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11:50–61
Robey IF, Baggett BK, Kirkpatrick ND, Roe DJ, Dosescu J, Sloane BF, Hashim AI, Morse DL, Raghunand N, Gatenby RA, Gillies RJ (2009) Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res 69:2260–2268
Acknowledgments
This study was financially supported by grants from Istituto Toscano Tumori and Ente Cassa di Risparmio di Firenze.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peppicelli, S., Bianchini, F., Torre, E. et al. Contribution of acidic melanoma cells undergoing epithelial-to-mesenchymal transition to aggressiveness of non-acidic melanoma cells. Clin Exp Metastasis 31, 423–433 (2014). https://doi.org/10.1007/s10585-014-9637-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-014-9637-6