Abstract
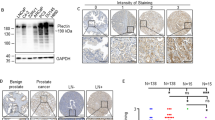
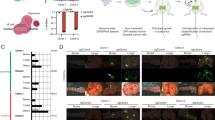
The non-canonical Wnt pathway, a regulator of cellular motility and morphology, is increasingly implicated in cancer metastasis. In a quantitative PCR array analysis of 84 Wnt pathway associated genes, both non-canonical and canonical pathways were activated in primary and metastatic tumors relative to normal prostate. Expression of the Wnt target gene PITX2 in a prostate cancer (PCa) bone metastasis was strikingly elevated over normal prostate (over 2,000-fold) and primary prostate cancer (over 200-fold). The elevation of PITX2 protein was also evident on tissue microarrays, with strong PITX2 immunostaining in PCa skeletal and, to a lesser degree, soft tissue metastases. PITX2 is associated with cell migration during normal tissue morphogenesis. In our studies, overexpression of individual PITX2A/B/C isoforms stimulated PC-3 PCa cell motility, with the PITX2A isoform imparting a specific motility advantage in the presence of non-canonical Wnt5a stimulation. Furthermore, PITX2 specific shRNA inhibited PC-3 cell migration toward bone cell derived chemoattractant. These experimental results support a pivotal role of PITX2A and non-canonical Wnt signaling in enhancement of PCa cell motility, suggest PITX2 involvement in homing of PCa to the skeleton, and are consistent with a role for PITX2 in PCa metastasis to soft and bone tissues. Our findings, which significantly expand previous evidence that PITX2 is associated with risk of PCa biochemical recurrence, indicate that variation in PITX2 expression accompanies and may promote prostate tumor progression and metastasis.






Similar content being viewed by others
References
Hall CL, Kang S, MacDougald OA, Keller ET (2006) Role of wnts in prostate cancer bone metastases. J Cell Biochem 97(4):661–672
Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET (2005) Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res 65(17):7554–7560
Glass DA 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation [see comment]. Dev Cell 8(5):751–764
Monroe DG, McGee-Lawrence ME, Oursler MJ, Westendorf JJ (2012) Update on Wnt signaling in bone cell biology and bone disease. Gene 492(1):1–18. doi:10.1016/j.gene.2011.10.044 gmr079 [pii]
Semenov MV, Habas R, MacDonald BT, He X (2007) Snapshot: noncanonical Wnt signaling pathways. Cell 131(7):1378
Veeman MT, Axelrod JD, Moon RT (2003) A second canon: functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell 5(3):367–377
Nishita M, Enomoto M, Yamagata K, Minami Y (2010) Cell/tissue-tropic functions of Wnt5a signaling in normal and cancer cells. Trends Cell Biol 20(6):346–354. doi:10.1016/j.tcb.2010.03.001 S0962-8924(10)00052-8 [pii]
De A (2011) Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai) 43(10):745–756. doi:10.1093/abbs/gmr079
Moustakas A, Heldin C-H (2007) Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci 98(10):1512–1520
Cox CJ, Espinoza HM, McWilliams B, Chappell K, Morton L, Hjalt TA, Semina EV, Amendt BA (2002) Differential Regulation of Gene Expression by PITX2 Isoforms. J Biol Chem 277(28):25001–25010. doi:10.1074/jbc.M201737200
Quentien MH, Barlier A, Franc JL, Pellegrini I, Brue T, Enjalbert A (2006) Pituitary transcription factors: from congenital deficiencies to gene therapy. J Neuroendocrinol 18(9):633–642
Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin CJ, Gleiberman A, Wang JB, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG (2002) Identification of a Wnt/DVI/beta-catenin—>Pitx2 pathway mediating cell-type-specific proliferation during development. Cell 111(5):673–685
Amendt BA, Sutherland LB, Russo AF (1999) Multifunctional role of the Pitx2 homeodomain protein C-terminal tail. Mol Cell Biol 19(10):7001–7010
Ai D, Liu W, Ma LJ, Dong FY, Lu MF, Wang DG, Verzi MP, Cai CL, Gage PJ, Evans S, Black BL, Brown NA, Martin JF (2006) Pitx2 regulates cardiac left-right asymmetry by patterning second cardiac lineage-derived myocardium. Dev Biol 296(2):437–449
Kurpios NA, Ibanes M, Davis NM, Lui W, Katz T, Martin JF, Belmonte JCI, Tabintt CJ (2008) The direction of gut looping is established by changes in the extracellular matrix and in cell: cell adhesion. Proc Natl Acad Sci USA 105(25):8499–8506
Ai D, Wang J, Amen M, Lu MF, Amendt BA, Martin JF (2007) Nuclear factor 1 and T-cell factor/LEF recognition elements regulate Pitx2 transcription in pituitary development. Mol Cell Biol 27(16):5765–5775
Amen M, Liu X, Vadlamudi U, Elizondo G, Diamond E, Engelhardt JF, Amendt BA (2007) PITX2 and beta-catenin interactions regulate lef-1 isoform expression. Mol Cell Biol 27(21):7560–7573
Baek SH, Kioussi C, Briata P, Wang DG, Nguyen HD, Ohgi KA, Glass CK, Wynshaw-Boris A, Rose DW, Rosenfeld MG (2003) Regulated subset of G(1) growth-control genes in response to derepression by the Wnt pathway. Proc Natl Acad Sci USA 100(6):3245–3250
Briata P, Ilengo C, Corte G, Moroni C, Rosenfeld MG, Chen CY, Gherzi R (2003) The Wnt/beta-catenin—>Pitx2 pathway controls the turnover of Pitx2 and other unstable mRNAs. Mol Cell 12(5):1201–1211
Vadlamudi U, Espinoza HM, Ganga M, Martin DM, Liu XM, Engelhardt JF, Amendt BA (2005) PITX2, beta-catenin and LEF-1 interact to synergistically regulate the LEF-1 promoter. J Cell Sci 118(6):1129–1137
Zhou WL, Lin LZ, Majumdar A, Li X, Zhang XX, Liu W, Etheridge L, Shi YQ, Martin J, Van de Ven W, Kaartinen V, Wynshaw-Boris A, McMahon AP, Rosenfeld MG, Evans SM (2007) Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGF beta 2. Nat Genet 39(10):1225–1234
Peng L, Dong G, Xu P, Ren LB, Wang CL, Aragon M, Zhou XD, Ye L (2010) Expression of Wnt5a in tooth germs and the related signal transduction analysis. Arch Oral Biol 55(2):108–114. doi:10.1016/j.archoralbio.2009.12.002 S0003-9969(09)00321-5 [pii]
Weiss G, Cottrell S, Distler J, Schatz P, Kristiansen G, Ittmann M, Haefliger C, Lesche R, Hartmann A, Corman J, Wheeler T (2009) DNA methylation of the PITX2 gene promoter region is a strong independent prognostic marker of biochemical recurrence in patients with prostate cancer after radical prostatectomy. J Urol 181(4):1678–1685
Vanaja DK, Ehrich M, Van den Boom D, Cheville JC, Karnes RJ, Tindall DJ, Cantor CR, Young CYF (2009) Hypermethylation of genes for diagnosis and risk stratification of prostate cancer. Cancer Invest 27(5):549–560
Banez LL, Sun L, van Leenders GJ, Wheeler TM, Bangma CH, Freedland SJ, Ittmann MM, Lark AL, Madden JF, Hartman A, Weiss G, Castanos-Velez E (2010) Multicenter clinical validation of PITX2 methylation as a prostate specific antigen recurrence predictor in patients with post-radical prostatectomy prostate cancer. J Urol 184(1):149–156. doi:10.1016/j.juro.2010.03.012
Goebel G, Auer D, Gaugg I, Schneitter A, Lesche R, Mueller-Holzner E, Marth C, Daxenbichler G (2011) Prognostic significance of methylated RASSF1A and PITX2 genes in blood- and bone marrow plasma of breast cancer patients. Breast Cancer Res Treat 130(1):109–117. doi:10.1007/s10549-010-1335-8
Fehm T, Banys M (2011) Circulating free DNA: a new surrogate marker for minimal residual disease? Breast Cancer Res Treat 130(1):119–122. doi:10.1007/s10549-011-1392-7
Dietrich D, Lesche R, Tetzner R, Krispin M, Dietrich J, Haedicke W, Schuster M, Kristiansen G (2009) Analysis of DNA methylation of multiple genes in microdissected cells from formalin-fixed and paraffin-embedded Tissues. J Histochem Cytochem 57(5):477–489
Nimmrich I, Sieuwerts AM, Gelder MEM, Schwope I, Vries JB, Harbeck N, Koenig T, Hartmann O, Kluth A, Dietrich D, Magdolen V, Portengen H, Look MP, Klijn JGM, Lesche R, Schmitt M, Maier S, Foekens JA, Martens JWM (2008) DNA hypermethylation of PITX2 is a marker of poor prognosis in untreated lymph node-negative hormone receptor-positive breast cancer patients. Breast Cancer Res Treat 111(3):429–437
Harbeck N, Nimmrich I, Hartmann A, Ross JS, Cufer T, Grutzmann R, Kristiansen G, Paradiso A, Hartmann O, Margossian A, Martens J, Schwope I, Lukas A, Muller V, Milde-Langosch K, Nahrig J, Foekens J, Maier S, Schmitt M, Lesche R (2008) Multicenter study using paraffin-embedded tumor tissue testing Pitx2 DNA methylation as a marker for outcome prediction in tamoxifen-treated, node-negative breast cancer patients. J Clin Oncol 26(31):5036–5042. doi:10.1200/jco.2007.14.1697
Span PN, Sieuwerts AM, Heuvel J, Spyratos F, Duffy MJ, Eppenberger-Castori S, Vacher S, O’Brien K, McKiernan E, Pierce A, Vuaroqueaux V, Foekens JA, Sweep F, Martens JWM (2009) Harmonisation of multi-centre real-time reverse-transcribed PCR results of a candidate prognostic marker in breast cancer: an EU-FP6 supported study of members of the EORTC—pathobiology group. Eur J Cancer 45(1):74–81
Roudier MP, True LD, Higano CS, Vesselle H, Ellis W, Lange P, Vessella RL (2003) Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol 34(7):646–653. doi:S0046817703001904 [pii]
Lai JS, Brown LG, True LD, Hawley SJ, Etzioni RB, Higano CS, Ho SM, Vessella RL, Corey E (2004) Metastases of prostate cancer express estrogen receptor-beta. Urology 64(4):814–820. doi:10.1016/j.urology.2004.05.036
Lamba P, Hjalt T, Bernard D (2008) Novel forms of paired-like homeodomain transcription factor 2 (PITX2): generation by alternative translation initiation and mRNA splicing. BMC Mol Biol 9(1):31
Saito K, Oku T, Ata N, Miyashiro H, Hattori M, Saiki I (1997) A modified and convenient method for assessing tumor cell invasion and migration and its application to screening for inhibitors. Biol Pharm Bull 20(4):345–348
Rosen S, Skaletsky HJ (2000) Primer 3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, pp 365–386
Wei Q, Adelstein RS (2002) Pitx2a expression alters actin-myosin cytoskeleton and migration of HeLa cells through Rho GTPase signaling. Mol Biol Cell 13(2):683–697
Gage PJ, Qian M, Wu DQ, Rosenberg KI (2008) The canonical Wnt signaling antagonist DKK2 is an essential effector of PITX2 function during normal eye development. Dev Biol 317(1):310–324
Vinarskaja A, Schulz WA, Ingenwerth M, Hader C, Arsov C (2011) Association of PITX2 mRNA down-regulation in prostate cancer with promoter hypermethylation and poor prognosis. Urol Oncol 31(5):621–627. doi:10.1016/j.urolonc.2011.04.010 S1078-1439(11)00139-6 [pii]
Shiratori H, Sakuma R, Watanabe M, Hashiguchi H, Mochida K, Sakai Y, Nishino J, Saijoh Y, Whitman M, Hamada H (2001) Two-step regulation of left–right asymmetric expression of Pitx2: initiation by nodal signaling and maintenance by Nkx2. Mol Cell 7(1):137–149. doi:S1097-2765(01)00162-9 [pii]
Holmberg J, Ingner G, Johansson C, Leander P, Hjalt TA (2008) PITX2 gain-of-function induced defects in mouse forelimb development. BMC Dev Biol 8:25
Shima Y, Zubair M, Komatsu T, Oka S, Yokoyama C, Tachibana T, Hjalt TA, Drouin J, Morohashi K (2008) Pituitary homeobox 2 regulates adrenal4 binding protein/steroidogenic factor-1 gene transcription in the pituitary gonadotrope through interaction with the intronic enhancer. Mol Endocrinol 22(7):1633–1646
Hall CL, Dubyk CW, Riesenberger TA, Shein D, Keller ET, van Golen K (2008) Type I collagen receptor (alpha(2)beta(1)) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia 10(8):797–803
Hayashi M, Maeda S, Aburatani H, Kitamura K, Miyoshi H, Miyazono K, Imamura T (2008) Pitx2 prevents osteoblastic transdifferentiation of myoblasts by bone morphogenetic proteins. J Biol Chem 283(1):565–571. doi:10.1074/jbc.M708154200
Zhu L, Marvin MJ, Gardiner A, Lassar AB, Mercola M, Stern CD, Levin M (1999) Cerberus regulates left–right asymmetry of the embryonic head and heart. Curr Biol 9(17):931–938
Suszko MI, Antenos M, Balkin DM, Woodruff TK (2008) Smad3 and Pitx2 cooperate in stimulation of FSH beta gene transcription. Mol Cell Endocrinol 281(1–2):27–36
Dietrich D, Hasinger O, Liebenberg V, Field JK, Kristiansen G, Soltermann A (2012) DNA methylation of the homeobox genes PITX2 and SHOX2 predicts outcome in non-small-cell lung cancer patients. Diagn Mol Pathol 21(2):93–104. doi:10.1097/PDM.0b013e318240503b
Basu M, Roy SS (2012) Wnt/β-catenin pathway is regulated by PITX2 homeodomain protein and thus contributes to the proliferation of human ovarian adenocarcinoma cell, SKOV-3. J Biol Chem. doi:10.1074/jbc.M112.409102
Fung FKC, Chan DW, Liu VWS, Leung THY, Cheung ANY, Ngan HYS (2012) Increased expression of PITX2 transcription factor contributes to ovarian cancer progression. PLoS One 7(5):e37076. doi:10.1371/journal.pone.0037076
Huang Y, Guigon CJ, Fan J, Cheng S-Y, Zhu G-Z (2010) Pituitary homeobox 2 (PITX2) promotes thyroid carcinogenesis by activation of cyclin D2. Cell Cycle 9(7):1333–1341
Aitchison AA, Veerakumarasivam A, Vias M, Kumar R, Hamdy FC, Neal DE, Milis IG (2008) Promoter methylation correlates with reduced Smad4 expression in advanced prostate cancer. Prostate 68(6):661–674
Connolly RM, Visvanathan K (2008) PITX2 DNA methylation: a potential prognostic or predictive biomarker in early breast cancer. Pharmacogenomics 9(12):1797–1798
Connolly RM, Visvanathan K (2008) PITX2 DNA methylation as a marker for outcome prediction in breast cancer patients receiving adjuvant tamoxifen. Pharmacogenomics 9(12):1798–1799
Maier S, Nimmrich I, Koenig T, Eppenberger-Castori S, Bohlmann I, Paradiso A, Spyratos F, Thomssen C, Mueller V, Naehrig J, Schittulli F, Kates R, Lesche R, Schwope I, Kluth A, Marx A, Martens JWM, Foekens JA, Schmitt M, Harbeck N (2007) DNA-methylation of the homeodomain transcription factor PITX2 reliably predicts risk of distant disease recurrence in tamoxifen-treated, node-negative breast cancer patients Technical and clinical validation in a multi-centre setting in collaboration with the European Organisation for Research and Treatment of Cancer (EORTC) PathoBiology group. Eur J Cancer 43(11):1679–1686
Massague J (2008) TGF beta in cancer. Cell 134(2):215–230
Wendt MK, Allington TM, Schiemann WP (2009) Mechanisms of the epithelial-mesenchymal transition by TGF-beta. Future Oncol 5(8):1145–1168. doi:10.2217/fon.09.90
Acknowledgments
We would like to thank the patients and their families who were willing to participate in the Prostate Cancer Donor Program, for without them research of this nature would not be possible. We would like to acknowledge Robert Vessella, and Lisha Brown and the rapid autopsy teams in the Urology Department at the University of Washington. This material is the result of work supported by resources from the VA Puget Sound Health Care System, Seattle, Washington, by the Pacific Northwest Prostate Cancer SPORE (P50CA97186), the PO1 NIH grant (PO1CA085859), and the Richard M. Lucas Foundation. CM is a recipient of the Career Development Award from the Pacific Northwest Prostate Cancer SPORE (P50CA097186). These studies were supported by the Australian National Health and Medical Research Council (Project Grant 455854), the UQ Diamantina Institute, Queensland Health, the Australasian Urologic Foundation, the Queensland Government Smart State PhD Funding Program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vela, I., Morrissey, C., Zhang, X. et al. PITX2 and non-canonical Wnt pathway interaction in metastatic prostate cancer. Clin Exp Metastasis 31, 199–211 (2014). https://doi.org/10.1007/s10585-013-9620-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-013-9620-7