Abstract
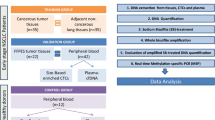
Free circulating DNA is increased in the serum/plasma of cancer patients, and methylation of certain genes has been found to be characteristic for malignancy. Therefore, we investigated the prognostic value of two promising genes, PITX2 and RASSF1A, in peripheral blood-plasma (PB-P) and bone marrow plasma (BM-P) of breast cancer patients. Peripheral blood and bone marrow samples from patients with primary breast cancer were prospectively collected during primary surgery at the Department of Obstetrics and Gynecology in Innsbruck (n = 428) from June 2000 to December 2006. The study has been approved by the ethical committee of the Medical University of Innsbruck. Methylation analysis was performed using MethyLight, a methylation-specific quantitative PCR-method. In univariate survival analysis, methylated PITX2 in PB-P was found to be a significant indicator for poor overall survival (OAS) and distant disease-free survival (DDFS) (P = 0.001 and P = 0.023). Methylated RASSF1A in PB-P was also an indicator for poor OAS and DDFS (P = 0.001 and P = 0.004). RASSF1A had also significant prognostic potential when determined in BM-P (P = 0.016). In multivariate survival analysis methylated PITX2 and RASSF1A in PB-P remained as therapy-independent prognostic factors for OAS (P = 0.021, P < 0.001). For DDFS only RASSF1A in PB-P showed prognostic significance (P = 0.002). Methylated RASSF1A and PITX2 in PB-P appear to have promising potential as prognostic markers in clinical use.

Similar content being viewed by others
References
Gormally E, Caboux E, Vineis P, Hainaut P (2007) Circulating free DNA in plasma or serum as biomarker of carcinogenesis: practical aspects and biological significance. Mutat Res Rev Mutat Res 635:105–117
Chen WY, Baylin SB (2005) Inactivation of tumor suppressor genes—choice between genetic and epigenetic routes. Cell Cycle 4:10–12
Donninger H, Vos MD, Clark GJ (2007) The RASSF1A tumor suppressor. J Cell Sci 120:3163–3172
Mehrotra J, Vali M, McVeigh M, Kominsky SL, Fackler MJ, Lahti-Domenici J, Polyak K, Sacchi N, Garrett-Mayer E, Argani P, Sukumar S (2004) Very high frequency of hypermethylated genes in breast cancer metastasis to the bone, brain, and lung. Clin Cancer Res 10:3104–3109
Dulaimi E, Hillinck J, de Caceres II, Al Saleem T, Cairns P (2004) Tumor suppressor gene promoter hypermethylation in serum of breast cancer patients. Clin Cancer Res 10:6189–6193
Muller HM, Widschwendter A, Fiegl H, Ivarsson L, Goebel G, Perkmann E, Marth C, Widschwendter M (2003) DNA methylation in serum of breast cancer patients: an independent prognostic marker. Cancer Res 63:7641–7645
Shukla S, Mirza S, Sharma G, Parshad R, Gupta SD, Ralhan R (2006) Detection of RASSF1A and RARbeta hypermethylation in serum DNA from breast cancer patients. Epigenetics 1:88–93
Fiegl H, Millinger S, Mueller-Holzner E, Marth C, Ensinger C, Berger A, Klocker H, Goebel G, Widschwendter M (2005) Circulating tumor-specific DNA: a marker for monitoring efficacy of adjuvant therapy in cancer patients. Cancer Res 65:1141–1145
Nimmrich I, Sieuwerts AM, Meijer-van Gelder ME, Schwope I, Bolt-de Vries J, Harbeck N, Koenig T, Hartmann O, Kluth A, Dietrich D, Magdolen V, Portengen H, Look MP, Klijn JG, Lesche R, Schmitt M, Maier S, Foekens JA, Martens JW (2008) DNA hypermethylation of PITX2 is a marker of poor prognosis in untreated lymph node-negative hormone receptor-positive breast cancer patients. Breast Cancer Res Treat 111:429–437
Maier S, Nimmrich I, Koenig T, Eppenberger-Castori S, Bohlmann I, Paradiso A, Spyratos F, Thomssen C, Mueller V, Naehrig J, Schittulli F, Kates R, Lesche R, Schwope I, Kluth A, Marx A, Martens JWM, Foekens JA, Schmitt M, Harbeck N (2007) DNA-methylation of the homeodomain transcription factor PITX2 reliably predicts risk of distant disease recurrence in tamoxifen-treated, node-negative breast cancer patients. Technical and clinical validation in a multi-centre setting in collaboration with the European Organisation for Research and Treatment of Cancer (EORTC) PathoBiology group. Eur J Cancer 43:1679–1686
Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin CJ, Gleiberman A, Wang JB, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG (2002) Identification of a Wnt/DVI/beta-catenin → Pitx2 pathway mediating cell-type-specific proliferation during development. Cell 111:673–685
Martin DM, Skidmore JM, Philips ST, Vieira C, Gage PJ, Condie BG, Raphael Y, Martinez S, Camper SA (2004) PITX2 is required for normal development of neurons in the mouse subthalamic nucleus and midbrain. Dev Biol 267:93–108
Pellegrini-Bouiller I, Manrique C, Gunz G, Grino M, Zamora AJ, Figarella-Branger D, Grisoli F, Jaquet P, Enjalbert A (1999) Expression of the members of the Ptx family of transcription factors in human pituitary adenomas. J Clin Endocrinol Metab 84:2212–2220
Semina EV, Reiter R, Leysens NJ, Alward WLM, Small KW, Datson NA, SiegelBartelt J, BierkeNelson D, Bitoun P, Zabel BU, Carey JC, Murray JC (1996) Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet 14:392–399
Stroun M, Maurice P, Vasioukhin V, Lyautey J, Lederrey C, Lefort F, Rossier A, Chen XQ, Anker P (2000) The origin and mechanism of circulating DNA. Ann N Y Acad Sci 906:161–168
Lee TH, Montalvo L, Chrebtow V, Busch MP (2001) Quantitation of genomic DNA in plasma and serum samples: higher concentrations of genomic DNA found in serum than in plasma. Transfusion 41:276–282
Nole F, Munzone E, Zorzino L, Minchella I, Salvatici M, Botteri E, Medici M, Verri E, Adamoli L, Rotmensz N, Goldhirsch A, Sandri MT (2008) Variation of circulating tumor cell levels during treatment of metastatic breast cancer: prognostic and therapeutic implications. Ann Oncol 19:891–897
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LWWM (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12:4218–4224
Koyanagi K, Mori T, O’Day SJ, Martinez SR, Wang HJ, Hoon DSB (2006) Association of circulating tumor cells with serum tumor-related methylated DNA in peripheral blood of melanoma patients. Cancer Res 66:6111–6117
Braun S, Pantel K, Muller P, Janni W, Hepp F, Kentenich CRM, Gastroph S, Wischnik A, Dimpfl T, Kindermann G, Riethmuller G, Schlimok G (2000) Cytokeratin-positive cells in the bone marrow, survival of patients with stage I, II, or III breast cancer. N Engl J Med 342:525–533
Braun S, Auer D, Marth C (2009) The prognostic impact of bone marrow micrometastases in women with breast cancer. Cancer Investig 27:598–603
Gerber B, Krause A, Muller H, Richter D, Reimer T, Makovitzky J, Herrnring C, Jeschke U, Kundt G, Friese K (2001) Simultaneous immunohistochemical detection of tumor cells in lymph nodes and bone marrow aspirates in breast cancer and its correlation with other prognostic factors. J Clin Oncol 19:960–971
Landys K, Persson S, Kovarik J, Hultborn R, Holmberg E (1998) Prognostic value of bone marrow biopsy in operable breast cancer patients at the time of initial diagnosis: results of a 20-year median follow-up. Breast Cancer Res Treat 49:27–33
Wiedswang G, Borgen E, Karesen R, Kvalheim G, Nesland JM, Schlichting HQE, Sauer T, Janbu J, Harbitz T, Naume B (2003) Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J Clin Oncol 21:3469–3478
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2005) Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 23:9067–9072
Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, Pierga JY, Marth C, Oruzio D, Wiedswang G, Solomayer EF, Kundt G, Strobl B, Fehm T, Wong GYC, Bliss J, Vincent-Salomon A, Pantel K (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353:793–802
Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, de Vijver MV, Wheeler TM, Hayes DF (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Borgen E, Naume B, Nesland J, Kvalheim G, Beiske K, Fodstad Ø, Diel I, Solomayer E, Theocharous P, Coombes R, Smith B, Wunder E, Marolleau J, Garcia J, Pantel K (1999) Standardization of the immunocytochemical detection of cancer cells in BM and blood: I. Establishment of objective criteria for the evaluation of immunostained cells. Cytotherapy 1:377–388
Strasak AM, Lang S, Kneib T et al (2009) Use of penalized splines in extended Cox-type additive hazard regression to flexibly estimate the effect of time-varying serum uric acid on risk of cancer incidence: a prospective, population-based study in 78,850 men. Ann Epidemiol 19:15–24
Umetani N, Giuliano AE, Hiramatsu SH, Amersi F, Nakagawa T, Martino S, Hoon DSB (2006) Prediction of breast tumor progression by integrity of free circulating DNA in serum. J Clin Oncol 24:4270–4276
Acknowledgment
This work was supported by the COMET Center ONCOTYROL.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Göbel, G., Auer, D., Gaugg, I. et al. Prognostic significance of methylated RASSF1A and PITX2 genes in blood- and bone marrow plasma of breast cancer patients. Breast Cancer Res Treat 130, 109–117 (2011). https://doi.org/10.1007/s10549-010-1335-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1335-8