Abstract
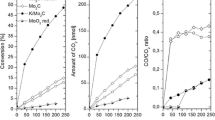
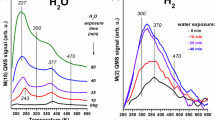
The photo-induced vapor-phase decomposition of formic acid was investigated on pure, N-doped and Rh-promoted TiO2. The bandgap of TiO2 was narrowed by 0.82–1.04 eV as a result of the incorporation N into TiO2. Adsorption of formic acid on pure TiO2 produced strong absorption bands due to formate species, the intensity of which decreased by illumination. The photodecomposition of formic acid on pure TiO2 at 300 K occurs to only a limited extent: on N-doped TiO2, however, it is enhanced by a factor of 2–4. The N-modified TiO2 catalyzes the photoreaction even in the visible light, which is attributed to the prevention of electron–hole recombination. The deposition of Rh on TiO2 markedly increased the extent of photodecomposition. The conversion is complete in 200 min, while the extent of decomposition reaches only ~30% on pure TiO2. The effect of Rh is explained by a better separation of charge carriers induced by illumination and by enhanced electron donation to the adsorbed formate species. On TiO2 samples both the dehydrogenation and dehydration reactions occurred, on Rh/TiO2 only a trace amount of CO was formed. Addition of water to formic acid eliminated this CO, but exerted no other influence on the occurrence the photoreaction.
Graphical Abstract
Effects of illumination time on the formation of CO2 and CO on pure and N-doped TiO2 in the photodecomposition of HCOOH.





Similar content being viewed by others
References
Sandstede G, Veziroglu TN, Derive C, Pottier J (1972) (eds) Proceedings of the ninth world hydrogen energy conference, Paris, p 1745
Haryanto A, Fernando S, Murali N, Adhikari S (2005) Energy & Fuels 19:2098
Brown LF (2001) Int J Hydrogen Energy 26:381
Ojeda M, Iglesia E (2009) Angew Chem Int Ed 48:4800
Koós Á, Solymosi F (2010) Catal Lett 138:23
Bulushev DA, Beloshapkin S, Ross JRH (2010) Catal Today 154:7
Gazsi A, Bánsági T, Solymosi F (2011) J Phys Chem C 115:15459
Solymosi F, Koós Á, Liliom N, Ugrai I (2011) J Catal 279:213
Halasi Gy, Ugrai I, Solymosi F (2011) J Catal 281:309
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Chem Rev 95:69
Linsebigler A, Lu G, Yates JT Jr (1995) Chem Rev 95:735
Muggli DS, Falconer JL (1999) J Catal 187:230
Chen T, Wu GP, Feng ZC, Hu GS, Su WG, Ying PL, Li C (2008) Chin J Catal 29:105
Zhang YJ, Zhang L (2009) Desalination 249:1017
Arana J, González Díaz O, Miranda Saracho M, Dona Rodríguez JM, Herrera Melián JA, Pérez Pena J (2001) Appl Catal B Env 32:49
Miller KL, Falconer JL, Medlin JW (2011) J Catal 278:321
Liao LF, Wu WC, Chen CY, Lin JL (2001) J Phys Chem B 105:7678
Miller KL, Lee CW, Falconer JL, Medlin JW (2010) J Catal 275:294
Beranek R, Kisch H (2008) Photochem Photobiol Sci 7:40
Xu J-H, Dai W-L, Li J, Cao Y, Li H, He H, Fan K (2008) Catal Commun 9:146
Pankove JI (1971) Optical processes in semiconductors. Prentience-Hall, New Jersey
Tang H, Prasad K, Sanilines R, Schmid PE, Levy F (1994) J Appl Phys 75:2042
Chang C-C, Wu W-C, Huang M-C, Huang I-C, Lin J-L (1999) J Catal 185:423
Busca G, Lamotte J, Lavalley JC, Lorenzelli V (1987) J Am Chem Soc 109:5197
Lavalley JC, Lamotte J, Busca G, Lorenzelli V (1986) J Chem Soc Chem Commun, pp 1006
Solymosi F, Pásztor M (1986) J Phys Chem 90:5312
Basu P, Panayotov D, Yates JT Jr (1987) J Phys Chem 91:3133
Berkó A, Ménesi G, Solymosi F (1996) J Phys Chem 100:17732
Solymosi F, Erdőhelyi A (1985) J Catal 91:327
Kecskés T, Raskó J, Kiss J (2004) Appl Catal A Gen 268:9
Sakthivel S, Shankar MV, Palanichamy M, Arabindoo B, Bahnemann DW, Murugesan V (2004) Water Res 38:3001
Hidalgo MC, Maicu M, Navío JA, Colón G (2008) Appl Catal B Env 81:49
Hidalgo MC, Maicu M, Navío JA, Colón G (2009) J Phys Chem C 113:12840
Solymosi F, Erdőhelyi A, Bánsági T (1981) J Catal 68:371
Tombacz I, Solymosi F (1994) Catal Lett 27:61
Szabó ZG, Solymosi F (1961) In: Proceedings of the 2nd international congress on catalysis, Technip, Paris, p 1627
Acknowledgments
This work was supported by the grant OTKA under Contract Number K 81517. A loan of rhodium chloride from Johnson–Matthey PLC and TiO2 (Hombicat) from Sachtleben is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Halasi, G., Schubert, G. & Solymosi, F. Photolysis of HCOOH over Rh Deposited on Pure and N-Modified TiO2 . Catal Lett 142, 218–223 (2012). https://doi.org/10.1007/s10562-011-0740-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-011-0740-x