Abstract
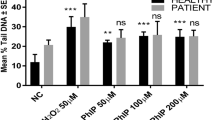
To account for the observed anticancer properties of plant polyphenols, we have earlier proposed a mechanism which involves the mobilization of endogenous copper ions by polyphenols leading to the generation of reactive oxygen species (ROS) that serve as proximal DNA cleaving agents and lead to cell death. Over the last decade we have proceeded to validate our hypothesis with considerable success. As a further confirmation of our hypothesis, in this paper we first show that oral administration of copper to rats leads to elevated copper levels in lymphocytes. When such lymphocytes with a copper overload were isolated and treated with polyphenols EGCG, genistein and resveratrol, an increased level of DNA breakage was observed. Further, preincubation of lymphocytes having elevated copper levels with the membrane permeable copper chelator neocuproine, resulted in inhibition of polyphenol induced DNA degradation. However, membrane impermeable chelator of copper bathocuproine, as well as iron and zinc chelators were ineffective in causing such inhibition in DNA breakage, confirming the involvement of endogenous copper in polyphenol induced cellular DNA degradation. It is well established that serum and tissue concentrations of copper are greatly increased in various malignancies. In view of this fact, the present results further confirm our earlier findings and strengthen our hypothesis that an important anticancer mechanism of plant polyphenols could be the mobilization of intracellular copper leading to ROS-mediated cellular DNA breakage. In this context, it may be noted that cancer cells are under considerable oxidative stress and increasing such stress to cytotoxic levels could be a successful anticancer approach.



Similar content being viewed by others
References
Ahmad MS, Fazal F, Rahman A, Hadi SM, Parish JH (1992) Activities of flavonoids for the cleavage of DNA in the presence of Cu(II): correlation with the generation of active oxygen species. Carcinogenesis 13:605–608
Ahmad A, Asad SF, Singh S, Hadi SM (2000) DNA breakage by resveratrol and Cu(II): reaction mechanism and bacteriophage inactivation. Cancer Lett 154:54–57
Ahmed N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H (1997) Green tea constituent epigallocatechin-3-gallate and induction of cell cycle arrest in human carcinoma cells. J Natl Cancer Inst 89:1881–1886
Ahsan H, Hadi SM (1998) Strand scission in DNA induced by curcumin in the presence of Cu(II). Cancer Lett 124:23–30
Apelgot S, Coppey J, Fromentin A, Guille E, Poupon MF, Roussel A (1986) Altered distribution of copper (64Cu) in tumor bearing mice and rats. Anticancer Res 6:159–164
Azam S, Hadi N, Khan NU, Hadi SM (2004) Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol In Vitro 18:555–561
Azmi AS, Bhat SH, Hadi SM (2005) Resveratrol-Cu(II) induced DNA breakage in human peripheral lymphocytes: implications for anticancer properties. FEBS Lett 579:3131–3135
Azmi AS, Bhat SH, Hanif S, Hadi SM (2006) Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: a putative mechanism for anticancer properties. FEBS Lett 580:533–538
Brewer G (2005) Anticopper therapy against cancer and diseases of inflammation and fibrosis. Drug Dis Today 10:1103–1109
Bryan SE (1979) Metal ions in biological systems. Marcel Dekker, New York
Carpentieri U, Myers J, Thorpe L, Daeschner CW, Haggard ME (1986) Copper, zinc and iron in normal and leukemic lymphocytes from children. Cancer Res 46:981–984
Chang KL, Cheng HL, Huang LW, Hseih BS et al (2008) Combined effects of terazosin and genistein on a metastatic, hormone-independent human prostate cancer cell line. Cancer Lett 276:14–20
Clement HV, Hirpara JL, Chawdhury SH, Pervaiz S (1998) Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signalling-dependent apoptosis in human tumor cells. Blood 92:996–1002
Czene S, Tiback M, Ringdahl MH (1997) pH-dependant DNA cleavage in permeabilized human fibroblasts. Biochem J 323:337–341
Devi GS, Prasad MH, Saraswathi I, Rao DN, Reddy PP (2000) Free radicals, antioxidant enzymes and lipid peroxidation in different types of leukemia. Clin Chim Acta 293:53–62
Ebadi E, Swanson S (1998) The status of zinc, copper and metallothionein in cancer patients. Prog Clin Biol Res 259:167–175
Ebara M, Fukuda H, Hanato R et al (2000) Relationship between zinc, copper and metallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. J Hepatol 33:415–422
Goodman VL, Brewer GJ, Merajver SD (2004) Copper deficiency as an anti-cancer strategy. Endocr Relat Cancer 11:255–263
Gupte A, Mumper RG (2008) Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev 35:32–46
Hadi SM, Asad SF, Singh S, Ahmad A (2000) A putative mechanism for anticancer and apoptosis inducing properties of plant-derived polyphenolic compounds. IUBMB Life 50:1–5
Hadi SM, Bhat SH, Azmi AS, Hanif S, Shamim U, Ullah MF (2007) Oxidative breakage of cellular DNA by plant polyphenols: a putative mechanism for anticancer properties. Semin Cancer Biol 17:370–376
Inoue M, Suzuki R, Koide T, Sakaguchi N, Ogihara Y, Yabu Y (1994) Antioxidant, gallic acid, induces apoptosis in HL60RG cells. Biochem Biophys Res Commun 204:898–904
Kagawa TF, Geierstanger BH, Wang AHJ, Ho PS (1991) Covalent modification of guanine bases in double stranded DNA: the 1:2-AZ-DNA structure of dc(CACACG) in the presence of CuCl2. J Biol Chem 266:20175–20184
Khan NS, Hadi SM (1998) Structural features of tannic acid important for DNA degradation in the presence of Cu(II). Mutagenesis 13:271–274
Kong Q, Beel JA, Lillehei KO (2000) A threshold concept for cancer therapy. Med Hypothesis 55:29–35
Kuo KW, Chen SF, Wu CC, Chen DR, Lee JH (2002) Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol Trace Elem Res 89:1–11
Malik A, Azam S, Hadi N, Hadi SM (2003) DNA degradation by water extract of green tea in the presence of copper ions: implications for anticancer properties. Phytother Res 17:358–363
Margalioth EJ, Udassin R, Cohen C, Maor J, Anteby SO, Schenker JG (1987) Serum copper level in gynaecologic malignancies. Am J Obstet Gynaecol 157:93–96
Moiseeva EP, Almeida GM, Jones GD, Manson MM (2007) Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells. Mol Cancer Ther 6:3071–3079
Nasulewis A, Mazur A, Opolski A (2004) Role of copper in angiogenesis: clinical-implication. J Trace Elem Med Biol 18:1–8
Park OJ, Surh YJ (2004) Chemopreventive potential of epigallocatechin gallate and genistein: evidence from epidemiological and laboratory studies. Toxicol Lett 150:43–56
Pervaiz S, Clement MV (2004) Tumor intracellular redox status and drug resistance-serendipity or a causal relationship? Curr Pharm Des 10:1969–1977
Pizzolo G, Savarin T, Molino AM, Ambrosette A, Todeschini G, Vettore L (1978) The diagnostic value of serum copper levels and other hematochemical parameters in malignancies. Tumorigenesis 64:55–61
Pool-Zoble BL, Guigas C, Klein RG, Neudecker CH, Renner HW, Schmezer P (1993) Assessment of genotoxic effects by lindane. Food Chem Toxicol 31:271–283
Powis G, Baker A (1997) Redox signaling and the control of cell growth and death. Adv Pharmacol 38:329–359
Rahman A, Shahabuddin A, Hadi SM, Parish JH, Ainley K (1989) Strand scission in DNA induced by quercetin and Cu(II): role of Cu(I) and oxygen free radicals. Carcinogenesis 10:1833–1839
Satoh K, Kodofuku T, Sakagami H (1997) Copper, but not iron, enhances apoptosis inducing activity of antioxidants. Anticancer Res 17:2487–2490
Schumacker PT (2006) Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10:175–176
Semczuk B, Pomykalski M (1973) Serum copper level in patients with laryngeal carcinoma. Otolaryngial Pol 27:17–23
Shamim U, Hanif S, Ullah MF, Azmi AS, Bhat SH, Hadi SM (2008) Plant polyphenols mobilize nuclear copper in human peripheral lymphocytes leading to oxidatively generated DNA breakage: implications for an anticancer mechanism. Free Radic Res 42:764–772
Singh NP, McCoy MT, Tice RR, Schneider EL (1998) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Swain J, Gutteridge JMC (1995) Prooxidant iron and copper, with ferroxidase and xanthine oxidase activities in human atherosclerotic material. FEBS Lett 368:513–515
Tice RR, Agurell E, Anderson D et al (2000) Single cell gel/Comet assay; guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H (2006) Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta phenylethyl isothiocyanate. Cancer Cell 10:241–252
Ullah MF, Shamim U, Hanif S, Azmi AS, Hadi SM (2009) Cellular DNA breakage by soy isoflavone genistein and its methylated structural analogue biochanin A. Mol Nutr Food Res 53:1376–1385
Ullah MF, Ahmad A, Zubair H, Khan HY et al (2011) Soy isoflavone genistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen species. Mol Nutr Food Res 55:553–559
US EPA (1994) Methods for determination of metals in environmental samples. Supplement I Washington, US Environmental Protection Agency, Office of Research and Development (EPA-600/R-94-111).
Vainio H, Weiderpress E (2006) Fruit and vegetables in cancer prevention. Nutr Cancer 54:111–142
Yoshida D, Ikada Y, Nakayama S (1993) Quantitative analysis of copper, zinc and copper/zinc ratio in selective human brain tumors. J Neurooncol 16:109–115
Zheng LF, Wei QY, Cai YJ, Fang JG, Zhou B, Yang L, Liu ZL (2006) DNA damage induced by resveratrol and its synthetic analogues in the presence of Cu(II) ions: mechanism and structure-activity relationship. Free Radic Biol Med 41:1807–1816
Zuo XL, Chen JM, Zhou X, Li XZ, Mei GY (2006) Levels of selenium, zinc, copper and antioxidant enzyme activity in patients with leukemia. Biol Trace Elem Res 114:41–54
Acknowledgments
The authors acknowledge the financial assistance provided by the University Grants Commission, New Delhi, under the DRS-II program and Junior Research Fellowship to HYK from CSIR, New Delhi.
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Husain Y. Khan and Haseeb Zubair are contributed equally.
Rights and permissions
About this article
Cite this article
Khan, H.Y., Zubair, H., Ullah, M.F. et al. Oral administration of copper to rats leads to increased lymphocyte cellular DNA degradation by dietary polyphenols: implications for a cancer preventive mechanism. Biometals 24, 1169–1178 (2011). https://doi.org/10.1007/s10534-011-9475-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-011-9475-9