Abstract
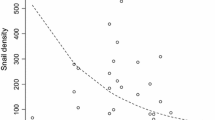
The pathogenic chytrid fungus Batrachochytrium dendrobatidis (Bd) is considered responsible for the population declines and extinctions of hundreds of amphibian species worldwide. The panzootic was likely triggered by human-assisted spread, but once the pathogen becomes established in a given region, its distribution is probably determined by local drivers. To assess the relative importance of potential drivers of infection in red-spotted newts (Notophthalmus viridescens), we measured Bd levels in 16 populations throughout central Pennsylvania. Infected individuals were detected in all but four populations, indicating that Bd is widespread in this region. We quantified local factors hypothesized to influence Bd, and found that infection levels were best predicted by the proportion of the pond substrate consisting of leaf litter or vegetation, along with a significant effect of water temperature. Bd infection in amphibians is temperature-dependent, and one possible explanation of the apparent substrate effect is that tree cover and vegetation provide shade, reducing the availability of shallow, warm-water patches in which newts might reduce or clear Bd infections. Alternatively, leaf litter and emergent vegetation might increase Bd infection more directly, perhaps by providing substrates for environmental growth of the fungus. We also observed a curvilinear relationship between Bd load and snout-vent length (a proxy for age), hinting that newts might develop acquired resistance to Bd infection. Though correlational, these results add to a growing body of evidence suggesting that environmental temperature is an important driver of Bd infection dynamics.


Similar content being viewed by others
References
Andre SE, Parker J, Briggs CJ (2008) Effect of temperature on host response to Batrachochytrium dendrobatidis infection in the mountain yellow-legged frog (Rana muscosa). Journal of Wildlife Diseases 44:716–720
Berger L, Speare R, Hines HB, Marantelli G, Hyatt AD, McDonald KR, et al. (2004) Effect of season and temperature on mortality in amphibians due to chytridiomycosis. Australian Veterinary Journal 82:434–439
Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Diseases of Aquatic Organisms 60:141–148
Campbell Grant EH, Bailey LL, Ware JL, Duncan KL (2008) Prevalence of the amphibian pathogen Batrachochytrium dendrobatidis in stream and wetland amphibians in Maryland, USA. Applied Herpetology 5:233–241
Crawley MJ (2007) The R Book, Chichester, UK: Wiley
Drake DL, Altig R, Grace JB, Walls SC (2007) Occurrence of oral deformities in larval anurans. Copeia 2007:449–458
Fisher MC, Garner TWJ (2007) The relationship between the emergence of Batrachochytrium dendrobatidis, the international trade in amphibians and introduced amphibian species. Fungal Biology Reviews 21:2–9
Fisher MC, Garner TWJ, Walker SF (2009) Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annual Review of Microbiology 63:291–310
Hossack BR, Muths E, Anderson CW, Kirshtein JD, Corn PS (2009) Distribution limits of Batrachochytrium dendrobatidis: a case study in the Rocky Mountains, USA. Journal of Wildlife Diseases 45:1198–1202
Hyatt AD, Boyle DG, Olsen V, Boyle DB, Berger L, Obendorf D, et al. (2007) Diagnostic assays and sampling protocols for the detection of Batrachochytrium dendrobatidis. Diseases of Aquatic Organisms 73:175–192
Jackman S (2008) pscl: classes and methods for R developed in the political science computational laboratory, Stanford University. Department of Political Science, Stanford University, Stanford, California. R package version 1.03. Available: http://pscl.stanford.edu
Johnson ML, Speare R (2005) Possible modes of dissemination of the amphibian chytrid Batrachochytrium dendrobatidis in the environment. Diseases of Aquatic Organisms 65:181–186
Kilpatrick AM, Briggs CJ, Daszak P (2010) The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends in Ecology & Evolution 25:109–118
Kluger MJ (1991) Fever: role of pyrogens and cryogens. Physiological Reviews 71:93–127
Kriger KM, Hero JM (2008) Altitudinal distribution of chytrid (Batrachochytrium dendrobatidis) infection in subtropical Australian frogs. Austral Ecology 33:1022–1032
Kriger KM, Hero JM, Ashton KJ (2006) Cost efficiency in the detection of chytridiomycosis using PCR assay. Diseases of Aquatic Organisms 71:149–154
Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J, et al. (2006) Emerging infectious disease and the loss of biodiversity in a neotropical amphibian community. Proceedings of the National Academy of Sciences of the United States of America 103:3165–3170
Lips KR, Mendelson JR, Munoz-Alonso A, Canseco-Marquez L, Mulcahy DG (2004) Amphibian population declines in montane southern Mexico: resurveys of historical localities. Biological Conservation 119:555–564
Longcore JE, Pessier AP, Nichols DK (1999) Batrachochytrium dendrobatidis gen et sp nov, a chytrid pathogenic to amphibians. Mycologia 91:219–227
Mitchell KM, Churcher TS, Garner TWJ, Fisher MC (2008) Persistence of the emerging pathogen Batrachochytrium dendrobatidis outside the amphibian host greatly increases the probability of host extinction. Proceedings of the Royal Society of London. Series B: Biological Sciences 275:329–334
Muths E, Corn PS, Pessier AP, Green DE (2003) Evidence for disease-related amphibian decline in Colorado. Biological Conservation 110:357–365
Muths E, Pilliod DS, Livo LJ (2008) Distribution and environmental limitations of an amphibian pathogen in the Rocky Mountains, USA. Biological Conservation 141:1484–1492
Nimon K, Roberts JK (2009) yhat: interpreting regression effects. R package version 1.0-3. Available: http://www.r-project.org
Nimon K, Lewis M, Kane R, Haynes RM (2008) An R package to compute commonality coefficients in the multiple regression case: an introduction to the package and a practical example. Behavioral Research Methods 40:457–466
Padgett-Flohr GE, Bommarito T, Sparling D (2007) Amphibian chytridiomycosis in commercially purchased research amphibians. Herpetological Review 38:390–393
Pearl CA, Bowerman J, Adams MJ, Chelgren ND (2009) Widespread occurrence of the chytrid fungus Batrachochytrium dendrobatidis on Oregon spotted frogs (Rana pretiosa). EcoHealth 6:209–218
Petranka JW (1998) Salamanders of the United States and Canada, Washington, DC: Smithsonian Institution Press
Picco AM, Collins JP (2008) Amphibian commerce as a likely source of pathogen pollution. Conservation Biology 22:1582–1589
Poole GC, Berman CH (2001) An ecological perspective on in-stream temperature: natural heat dynamics and mechanisms of human-caused thermal degradation. Environmental Management 27:787–802
R Development Core Team (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available: http://www.R-project.org
Rachowicz LJ, Briggs CJ (2007) Quantifying the disease transmission function: effects of density on Batrachochytrium dendrobatidis transmission in the mountain yellow-legged frog Rana muscosa. Journal of Animal Ecology 76:711–721
Rachowicz LJ, Knapp RA, Morgan JAT, Stice MJ, Vredenburg VT, Parker JM, et al. (2006) Emerging infectious disease as a proximate cause of amphibian mass mortality. Ecology 87:1671–1683
Rachowicz LJ, Vredenburg VT (2004) Transmission of Batrachochytrium dendrobatidis within and between amphibian life stages. Diseases of Aquatic Organisms 61:75–83
Raffel TR (2006) Causes and consequences of seasonal dynamics in the parasite community of red-spotted newts (Notophthalmus viridescens). PhD dissertation. Penn State University, University Park, PA
Raffel TR, Dillard JR, Hudson PJ (2006a) Field evidence for leech-borne transmission of amphibian Ichthyophonus. Journal of Parasitology 92:1256–1264
Raffel TR, Rohr JR, Kiesecker JM, Hudson PJ (2006b) Negative effects of changing temperature on amphibian immunity under field conditions. Functional Ecology 20:819–828
Raffel TR, Bommarito T, Barry DS, Witiak SM, Shackelton LA (2008) Widespread infection of the Eastern red-spotted newt (Notophthalmus viridescens) by a new species of Amphibiocystidium, a genus of fungus-like mesomycetozoan parasites not previously reported in North America. Parasitology 135:1–13
Raffel TR, LeGros RP, Love BC, Rohr JR, Hudson PJ (2009) Parasite age-intensity relationships in red-spotted newts: does immune memory influence salamander disease dynamics? International Journal for Parasitology 39:231–241
Raffel TR, Lloyd-Smith JO, Sessions SK, Hudson PJ, Rohr JR (in press) Does the early frog catch the worm? Disentangling potential drivers of a parasite age-intensity relationship in tadpoles. Oecologia
Ramsey JP, Reinert LK, Harper LK, Woodhams DC, Rollins-Smith LA (2010) Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infection and Immunity 78:3981–3992
Richards-Zawacki CL (2010) Thermoregulatory behaviour affects prevalence of chytrid fungal infection in a wild population of Panamanian golden frogs. Proceedings of the Royal Society of London. Series B: Biological Sciences 277:519–528
Rodder D, Veith M, Lotters S (2008) Environmental gradients explaining the prevalence and intensity of infection with the amphibian chytrid fungus: the host’s perspective. Animal Conservation 11:513–517
Rohr JR, Raffel TR (2010) Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proceedings of the National Academy of Sciences of the United States of America 107:8269–8274
Rohr JR, Raffel TR, Romansic JM, McCallum H, Hudson PJ (2008) Evaluating the links between climate, disease spread, and amphibian declines. Proceedings of the National Academy of Sciences of the United States of America 105:17436–17441
Rose C, Crumpton WG (1996) Effects of emergent macrophytes on dissolved oxygen dynamics in a prairie pothole wetland. Wetlands 16:495–502
Rothermel BB, Walls SC, Mitchell JC, Dodd CK, Irwin LK, Green DE, et al. (2008) Widespread occurrence of the amphibian chytrid fungus Batrachochytrium dendrobatidis in the southeastern USA. Diseases of Aquatic Organisms 82:3–18
Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, et al. (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4:125–134
Stice MJ, Briggs CJ (2010) Immunization is ineffective at preventing infection and mortality due to the amphibian chytrid fungus Batrachochytrium dendrobatidis. Journal of Wildlife Diseases 46:70–77
Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues AS, Fischman DL, et al. (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306:1783–1786
Venables WN, Ripley BD (2002) Modern Applied Statistics with S, 4th ed., New York: Springer
Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, et al. (2009) Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines. Science 326:582–585
Wareing MD, Tannock GA (2001) Live attenuated vaccines against influenza; an historical review. Vaccine 19:3320–3330
Weldon C, du Preez LH, Hyatt AD, Muller R, Speare R (2004) Origin of the amphibian chytrid fungus. Emerging Infectious Diseases 10:2100–2105
Woodhams DC, Alford RA, Marantelli G (2003) Emerging disease of amphibians cured by elevated body temperature. Diseases of Aquatic Organisms 55:65–67
Woodhams DC, Ardipradja K, Alford RA, Marantelli G, Reinert LK, Rollins-Smith LA (2007) Resistance to chytridiomycosis varies among amphibian species and is correlated with skin peptide defenses. Animal Conservation 10:409–417
Zeileis A, Hothorn T (2002) Diagnostic checking in regression relationships. R News 2:7–10
Zeileis A, Kleiber C, Jackman S (2008) Regression models for count data in R. Journal of Statistical Software 27:1–25
Acknowledgments
We thank the members of the Hudson and Rohr labs for their advice and support. In particular, J. Dillard provided extensive assistance with the survey, newt collections, and dissections; J. Romansic helped to develop the qPCR methods; and M. McCoy suggested the use of zero-inflated negative binomial glm for analysis of the Bd data. A. Marm Kilpatrick and two anonymous reviewers provided valuable comments that improved the paper. This work was supported by a NSF Fellowship (to T.R.R.), NSF Dissertation Improvement Grant 0508847 (to T.R.R.), and US Environmental Protection Agency Science to Achieve Results Grant R833835 (to J.R.R and T.R.R.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raffel, T.R., Michel, P.J., Sites, E.W. et al. What Drives Chytrid Infections in Newt Populations? Associations with Substrate, Temperature, and Shade. EcoHealth 7, 526–536 (2010). https://doi.org/10.1007/s10393-010-0358-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10393-010-0358-2