Abstract
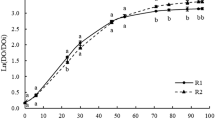
Large-scale production has been the major obstacle to the success of many biopesticides. The spreading of microbial biocontrol agents against postharvest disease, as a safe and environmentally friendly alternative to synthetic fungicides, is quite dependent on their industrial mass production from low-cost raw materials. Considerable interest has been shown in using agricultural waste products and by-products from food industry as nitrogen and carbon sources. In this work, carob pulp aqueous extracts were used as carbon source in the production of the biocontrol agent Pantoea agglomerans PBC-1. Optimal sugar extraction was achieved at a solid/liquid ratio of 1:10 (w/v), at 25°C, for 1 h. Batch experiments were performed in shake flasks, at different concentrations and in stirred reactors at two initial inoculums concentrations, 106 and 107 cfu ml−1. The initial sugar concentration of 5 g l−1 allowed rapid growth (0.16 h−1) and high biomass productivity (0.28 g l−1 h−1) and was chosen as the value for use in stirred reactor experiments. After 22 and 32 h of fermentation the viable population reached was 3.2 × 109 and 6.2 × 109 cfu ml−1 in the fermenter inoculated at 106 cfu ml−1 and 2.7 × 109 and 6.7 × 109 cfu ml−1 in the bioreactor inoculated at 107 cfu ml−1. A 78% reduction of the pathogen incidence was achieved with PBC-1 at 1 × 108 cfu ml−1, grown in medium with carob extracts, on artificially wounded apples stored after 7 days at 25°C against P. expansum.



Similar content being viewed by others
References
Abadias M, Teixidó N, Usall J, Viñas I (2003) Optimization of growth conditions of the postharvest biocontrol agent Candida sake CPA-1 in a lab-scale fermenter. J Appl Microbiol 95:301–309. doi:10.1046/j.1365-2672.2003.01976.x
Anselmo A, Cabral JMS, Novais JM (1989) The adsorption of Fusarium flocciferum spores on celite particles and their use in the degradation of phenol. Appl Microbiol Biotechnol 31:200–203. doi:10.1007/BF00262463
Avallone R, Plessi M, Baraldi A, Monzani M (1997) Determination of chemical composition of carob (Ceratonia siliqua): protein, fat, carbohydrates and tannins. J Food Compos Anal 10:166–172. doi:10.1006/jfca.1997.0528
Ayaz FA, Torun H, Ayaz S, Correia PJ, Alaiz M, Sanz C, Gruz J, Strnad M (2007) Determination of chemical composition of anatolian carob pod (Ceratonia siliqua L.): sugars, amino and organic acids, minerals and phenolic compounds. J Food Qual 30:1040–1055. doi:10.1111/j.1745-4557.2007.00176.x
Batlle I, Tous J (1997) Carob tree. Ceratonia siliqua L. promoting the conservation and use of underutilized and neglected crops. 17. Institute of Plant Genetics and Crop Plant Research. Gatersleben/International Plant Genetic Resources Institute, Rome
Biner B, Gubbuk H, Karhanb M, Aksub M, Pekmezci M (2007) Sugar profiles of the pods of cultivated and wild types of carob bean (Ceratonia siliqua L.) in Turkey. Food Chem 100:1453–1455. doi:10.1016/j.foodchem.2005.11.037
Bonaterra A, Mari M, Casalimi L, Montesinos E (2003) Biological control of Monilinia laxa and Rhizopus stolonifer in postharvest of stone fruit by Pantoea agglomerans EPS125 and putative mechanisms of antagonism. Int J Food Microbiol 84:93–104. doi:10.1016/S0168-1605(02)00403-8
Braun-Kiewnick A, Jacobsen BJ, Sands DC (2000) Biological control of Pseudomonas syringae pv. syringae, the causal agent of basal kernel blight of barley, by antagonistic Pantoea agglomerans. Phytopathology 90:368–375
Choi M, Park Y (2003) Production of yeast biomass using waste Chinese cabbage. Biomass Bioenergy 25:221–226. doi:10.1016/S0961-9534(02)00194-0
Correia PJ, Martins-Loução MA (2005) The use of macronutrients and water in marginal mediterranean areas: the case of carob-tree. Filed Crops Res 91:1–6. doi:10.1016/j.fcr.2004.05.004
Costa E, Teixidó N, Usall J, Atarés E, Viñas I (2001) Production of the biocontrol agent Pantoea agglomerans CPA-1 using commercial products and by-products. Appl Microbiol Biotechnol 56:367–371. doi:10.1007/s002530100666
Craig WJ, Nguyen TT (2006) Caffeine and theobromine levels in cocoa and carob products. J Food Sci 49:302–303. doi:10.1111/j.1365-2621.1984.tb13737.x
Droby S (2006) Biological control of postharvest diseases of fruits and vegetables: difficulties and challenges. Phytopathol Pol 39:105–117
Droby S (2009) Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol Technol 52:137–145. doi:10.1016/j.postharvbio.2008.11.009
Eckert JW, Ogawa JM (1988) The chemical control of postharvest diseases deciduous fruits, berries, vegetables and root/tuber crops. Annu Rev Phytopathol 26:433–469. doi:10.1146/annurev.py.26.090188.002245
Galaction AI, Cascaval D, Oniscu C, Turnea M (2004) Enhancement of oxygen mass transfer in stirred bioreactors using oxygen-vectors. 1. Simulated fermentation broths. Bioprocess Biosyst Eng 26:231–238. doi:10.1007/s00449-004-0353-5
Garcia-Ochoa F, Gomez E (2009) Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol Adv 27:153–176. doi:10.1016/j.biotechadv.2008.10.006
Gomes N, Aguedo M, Teixeira J, Belo I (2007) Oxygen mass transfer in a biphasic medium: influence on the biotransformation of methyl ricinoleate into γ-decalactone by the yeast Yarrowia lipolytica. Biochem Eng J 35:380–386. doi:10.1016/j.bej.2007.02.002
Henis Y, Tagari H, Volcani R (1964) Effect of water extracts of carob pods tannic acid their derivatives on morphology growth of microorganisms. Appl Environ Microbiol 12:204–209
Hofstein R, Fridlender B, Chalutz E, Droby S (1994) Large scale production and pilot testing of biocontrol agents of postharvest diseases. In: Wilson CL, Wisniewski ME (eds) Biological control of postharvest diseases of fruits and vegetables-theory and practice. CRC, Boca Raton, pp 89–100
Hsieh TF, Huang HC, Erickson RS (2005) Biological control of bacterial wilt of bean using a bacterial endophyte, Pantoea agglomerans. J Phytopathol 153:608–614
Janisiewicz WJ (1998) Biocontrol of postharvest diseases of temperate fruits. Challenges and opportunities. In: Boland G, Kuykendall LD (eds) Plant-microbe interactions and biological control. Marcel Dekker, New York, pp 171–198
Janisiewicz WJ, Korsten L (2002) Biological control of postharvest disease on fruits. Annu Rev Phytopathol 40:411–441. doi:10.1146/annurev.phyto.40.120401.130158
Janisiewicz WJ, Tworkoski TJ, Sharer C (2000) Characterizing the mechanism of biological control postharvest disease on fruits a simple method to study competition for nutrient. Phytopatol 90:1196–1200
Makris DP, Kefalas P (2004) Carob pods (Ceratonia siliqua L.) as a source of polyphenolic antioxidants. Food Technol Biotechnol 42:105–108
Manso T, Nunes C, Raposo S, Lima-Costa ME (2010) Production of the biocontrol agent Pantoea agglomerans PBC-1 in a stirred tank reactor by batch and fed-batch cultures. World J Microbiol Biotechnol 26:725–735. doi:10.1007/s11274-009-0229-6
Nagy E, Feczkó T, Koroknai B (2007) Enhancement of oxygen mass transfer rate in the presence of nanosized particles. Chem Eng Sci 62:7391–7398. doi:10.1016/j.ces.2007.08.064
Nunes C, Usall J, Teixidó N, Viñas I (2001) Biological control of postharvest pear diseases using a bacterium, Pantoea agglomerans CPA-2. Int J Food Microbiol 70:53–61
Nunes C, Usall J, Teixidó N, Fons E, Viñas I (2002) Post-harvest biological control by Pantoea agglomerans (CPA-2) on Golden Delicious apples. J Appl Microbiol 92:247–255
Nunes C, Manso T, Lima-Costa ME (2009) Postharvest biological control of citrus fruit. Tree For Sci Biotechnol 3:116–126
Olle B, Bucak S, Holmes TC, Bromberg L, Hatton TA, Wang DIC (2006) Enhancement of oxygen mass transfer using functionalized magnetic nanoparticles. Ind Eng Chem Res 45:4355–4363. doi:10.1021/ie051348b
Peighami-Ashnaei S, Sharifi-Tehrani A, Ahmadzadeh M, Behboudi K (2009) Interaction of different media on production and biocontrol efficacy of Pseudomonas flurescens P-35 and Bacillus subtilis B-3 against grey mould apple. J Plant Pathol 91:65–70
Peris-Tortajada M (2000) HPLC determination of carbohydrates in food. In: Nollet LML (ed) Food analyses by HPLC, 2nd edn. Marcel Dekker, Inc, New York, pp 287–302
Petit MD, Pinilla JM (1995) Production and purification of a sugar syrup from carob pods. Food Sci Technol-Lebensmittel-Wissenschaft Technologie 28:145–152. doi:10.1016/S0023-6438(95)80027-1
Raposo S, Pardao JM, Diaz I, Lima-Costa ME (2009) Kinetic modelling of bioethanol production using agro-industrial by-products. Int J Energy Environ 1(3):1–8
Roseiro JC, Girio FM, Colaço MTA (1991) The influence of storage stability on the use of carob pulp aqueous extract as raw material for fermentation processes. Food Sci Technol-Lebensmittel-Wissenschaft Technologie 24:508–512
Roseiro JC, Girio FM, Collaço MTA (1991) Yield improvements in carob sugar extraction. Process Biochem 26:179–182
Roukas T (1996) Ethanol production from non-sterilized beet molasses by free and immobilized Saccharomyces cerevisiae cells using fed-batch culture. J Food Eng 27:87–96. doi:10.1016/0260-8774(94)00076-L
Roukas T (1999) Citric acid production from carob pod by solid-state fermentation. Enzyme Microbial Technol 24:54–59. doi:10.1016/S0141-0229(98)00092-1
Santos M, Rodrigues A, Teixeira JA (2005) Production of dextran and fructose from carob pod extract and cheese whey by Leuconostoc mesenteroides NRRL B512(f). Biochem Eng J 25:1–6. doi:10.1016/j.bej.2005.01.022
Spots RA, Cervantes LA (1986) Population pathogenecity and benomyl resistant of Botrytis spp. Penicillium spp. and Mucor piriformis in packing houses. Plant Dis 70:106–108. doi:10.1094/PD-70-106
Teixidó N, Usall J, Nunes C, Torres R, Abadias M and Viñas I (2009) Preharvest strategies to control postharvest diseases in fruits. In: Prusky D, Gullino ML (eds) Post-harvest pathology. Plant pathology in the 21st century, vol 2. Springer, New York, pp 89–106. doi:10.1007/978-1-4020-8930-5_7
Trotel-Aziz P, Couderchet M, Biagianti S, Aziz A (2008) Characterization of new bacterial biocontrol agents Acinetobacter, Bacillus, Pantoea and Pseudomonas spp. Penicillium spp. and Mucor piriformis in packing houses. Environ Exp Bot 64:21–32. doi:10.1016/j.envexpbot.2007.12.009
Turhan I, Tetik N, Aksu M, Karhan M, Certel M (2006) Liquid–solid extraction of soluble solids and total phenolic compounds of carob bean (Ceratonia siliqua L.). J Food Process Eng 29:498–507. doi:10.1111/j.1745-4530.2006.00078.x
Usall J, Teixidó N, Abadias M, Torres R, Cañamas T, Viñas I (2009) Improving formulation of biocontrol agents manipulating production process. In: Prusky D, Gullino ML (eds) Post-harvest pathology. Plant pathology in the 21st century, vol 2. Springer, New York, pp 149–169. doi:10.1007/978-1-4020-8930-5_11
Verma M, Brar SK, Tyagi RD, Surampalli RY, Valero JR (2007) Industrial wastewaters and dewatered sludge: rich nutrient source for production and formulation of biocontrol agent, Trichoderma viride. World J Microbiol Biotechnol 23:1695–1703. doi:10.1007/s11274-007-9417-4
Wisniewski ME, Wilson CL (1992) Biological control of postharvest diseases of fruits and vegetables: recent advances. HortScience 27:94–98
Yousif AK, Alghzawi HM (2000) Processing and characterization of carob powder. Food Chem 69:283–287. doi:10.1016/S0308-8146(99)00265-4
Zhang Z, Jin B, Kelly JM (2007) Production of lactic and byproducts from waste potato starch by Rhizopus arrhizus: role of nitrogen sources. World J Microbiol Biotechnol 23:229–236. doi:10.1007/s11274-006-9218-1
Zimmermann HF, Anderlei T, Büchs J, Binder M (2006) Oxygen limitation is a pitfall during screening for industrial strains. Appl Microbiol Biotechnol 72:1157–1160. doi:10.1007/s00253-006-0414-6
Acknowledgments
The present work was conducted in the frame of a doctorate scholarship awarded to Teresa Manso (SFHR/BD/21922/2005) and supported by the project POCTI/AGR/45098/2002, by the Fundação para a Ciência e a Tecnologia, Ministério da Ciência e Superior. We also thank AGRUPA (Industrial Association of Carob and Almond Producers, C.R.L.) for kindly supplying carob kibbles.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manso, T., Nunes, C., Raposo, S. et al. Carob pulp as raw material for production of the biocontrol agent P. agglomerans PBC-1. J Ind Microbiol Biotechnol 37, 1145–1155 (2010). https://doi.org/10.1007/s10295-010-0762-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-010-0762-1