Abstract
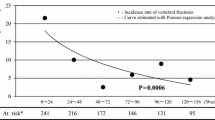
An open-label study with blinded evaluation was performed to compare the preventive effect of a calcium supplement alone (monotherapy) or calcium supplement plus menatetrenone (combined therapy) on fracture in osteoporotic postmenopausal women aged 50 years or older. Patients were randomized to receive monotherapy (n = 2,193) or combined therapy (n = 2,185). Before randomization, the subjects were stratified into a subgroup without vertebral fractures (n = 2,986; no-fracture subgroup) and a subgroup with at least one vertebral fracture (n = 1,392; fracture subgroup). The incidence rate of new vertebral fractures during 36 months of treatment (primary endpoint) did not differ significantly between either subgroup of the two treatment groups. Although the cumulative 48-month incidence rate of new clinical fractures (secondary endpoint) was lower in the combined therapy group, the difference was not significant. There was a lower risk of new vertebral fractures in patients with at least five baseline fractures who received combined therapy. Also, the loss of height was less with combined therapy than with monotherapy among patients 75 years of age or older at enrollment, those whose last menstrual period occurred 30 years or more before enrollment, and those with at least five vertebral fractures at enrollment. Adverse events and adverse reactions were more frequent in the combined therapy group. In conclusion, menatetrenone therapy was not effective for preventing vertebral fractures in the full analysis set of this study, but the results suggested that it may prevent vertebral fractures in patients with more advanced osteoporosis.




Similar content being viewed by others
References
Bouckaert JH, Said AH (1960) Fracture healing by vitamin K. Nature (Lond) 185:849
Stenflo J, Fernlund P, Egan W, Roepstorff P (1974) Vitamin K dependent modification of glutamic acid residues in prothrombin. Proc Natl Acad Sci USA 71:2730–2733
Hauschka PV, Lian JB, Gallop PM (1975) Direct identification of the calcium-binding amino acid, γ-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci USA 72:3925–3929
Price PA, Otsuka AS, Poser JW, Kristaponis J, Raman N (1976) Characterization of a γ-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci USA 73:1447–1451
Hara K, Akiyama Y, Nakamura T, Murota S, Morita I (1995) The inhibitory effect of vitamin K2 (menatetrenone) on bone resorption may be related to its side chain. Bone (NY) 16:179–184
Koshihara Y, Hoshi K (1997) Vitamin K2 enhances osteocalcin accumulation in the extracellular matrix of human osteoblasts in vitro. J Bone Miner Res 12:431–438
Hart JP, Shearer MJ, Klenerman L, Catterall A, Reeve J et al (1985) Electrochemical detection of depressed circulating levels of vitamin K1 in osteoporosis. J Clin Endocrinol Metab 60:268–1269
Bitensky L, Hart JP, Catterall A, Hodges SJ, Pilkington MJ et al (1988) Circulating vitamin K levels in patients with fractures. J Bone Joint Surg 70:663–664
Hodges SJ, Akesson K, Vergnaud P, Obrant K, Delmas PD (1993) Circulating levels of vitamin K1 and K2 decreased in elderly women with hip fracture. J Bone Miner Res 10:1241–1245
Szulc P, Chapuy MC, Meunier PJ, Delmas PD (1993) Serum undercarboxylated osteocalcin is a marker of the risk of hip fracture in elderly women. J Clin Invest 91:1769–1774
Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S et al (2001) Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab 19:331–337
Inoue T, Kushida K, Miyamoto S, Sumi Y, Orimo H et al (1983) Quantitative assessment of bone density on X-ray picture. J Jpn Orthop Assoc 57:1923–1936
Orimo H, Fujita T, Onomura T, Inoue T, Kushida K et al (1992) Clinical evaluation of Ea-0167 (menatetrenone) in the treatment of osteoporosis: phase III double-blind multicenter comparative study with alfacalcidol. Clin Eval 20:45–100
Rothman KJ, Greenland S (1998) Modern epidemiology, 2nd edn. Lippincott-Raven, Philadelphia
Orimo H, Shiraki M, Hasyashi Y, Hoshino T, Onaya T et al (1994) Effects of 1α-hydroxyvitamin D3 on lumbar bone mineral density and vertebral fractures in patients with postmenopausal osteoporosis. Calcif Tissue Int 54:370–376
Tilyard MW, Spears GFS, Thomson J, Dovey S (1992) Treatment of postmenopausal osteoporosis with calcitriol or calcium. N Engl J Med 326:357–362
Aloia JF, Vaswani A, Yeh JK, Ellis K, Yasumura S et al (1988) Calcitriol in the treatment of postmenopausal osteoporosis. Am J Med 84:401–408
Gallagher JC, Riggs BL, Recker RR, Goldgar D (1989) The effect of calcitriol on patients with postmenopausal osteoporosis with special reference to fracture frequency (42922). Proc Soc Exp Biol 191:287–292
The European Prospective Osteoporosis Study (EPOS) Group (2002) Incidence of vertebral fracture in Europe: results from the European prospective osteoporosis study (EPOS). J Bone Miner Res 17:716–724
Fujiwara S, Kasagi F, Masunari N, Naito K, Suzuki G et al (2003) Fracture prediction from bone mineral density in Japanese men and women. J Bone Miner Res 18:1547–1553
Hayashi Y, Fujita T, Inoue T (1992) Decrease of vertebral fracture in osteoporotics by administration of 1α-hydroxy-vitamin D3. J Bone Miner Metab 10:184–188
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 282:1344–1352
Reginster J-Y, Minne HW, Sorensen OH, Hooper M, Roux C et al (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 11:83–91
Cockayne S, Adamson J, Lanham-New S, Shearer M, Gilbody S et al (2006) Vitamin K and the prevention of fractures. Arch Intern Med 166:1256–1261
Knapen MHJ, Schurgers LJ, Vermeer C (2007) Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int 18:963–972
Kushida K, Fukunaga M, Kishimoto H, Shiraki M, Itabashi A et al (2004) A comparison of incidences of vertebral fracture in Japanese patients with involutional osteoporosis treated with risedronate and etidronate: a randomized, double-masked trial. J Bone Miner Metab 22:469–478
Plantalech L, Guillaumont M, Vergnaud P, Leclercq M, Delmas PD (1991) Impairment of gamma carboxylation of circulating osteocalcin (bone gla protein) in elderly women. J Bone Miner Res 6:1211–1216
Tsugawa N, Shiraki M, Suhara Y, Kamao M, Tanaka K et al (2006) Vitamin K status of healthy Japanese women: age-related vitamin K requirement for γ-carboxylation of osteocalcin. Am J Clin Nutr 83:380–386
Ross PD, Fujiwara S, Huang C, Davis JW, Epstein RS et al (1995) Vertebral fracture prevalence in women in Hiroshima compared to Caucasians or Japanese in the US. Int J Epidemiol 24:1171–1177
Jinbayashi H, Aoyagi K, Ross PD, Ito M, Shindo H et al (2002) Prevalence of vertebral deformity and its associations with physical impairment among Japanese women: the Hizen-Oshima study. Osteoporos Int 13:723–730
Lipid Research Clinical Program (1984) The lipid research clinical coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA 251:351–364
Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y et al (2006) Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomized controlled trial. Lancet 368:1155–1163
Acknowledgments
Research funds were provided by the Japanese Ministry of Health, Labor and Welfare for the first 2 years of the study, and thereafter the study was funded by Eisai Co. Ltd. (Tokyo, Japan). We thank all the study participants, physicians, and co-medical staff.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The following individuals and organizations (chairmen are indicated by asterisks) were responsible for the design, execution, analysis, and reporting of this study: Steering/Management Committee (Tetsuo Inoue*, Hideaki Takahashi, Hiroshi Minaguchi, Hirotoshi Morii, Kaoru Yamazaki, Keiro Ono, Kichizo Yamamoto, Kiyoshi Kaneda, Shoichi Kokubun, Tadashi Funakoshi, Toshiharu Fujita, Toshitaka Nakamura, Tosiya Sato, and Yukihide Iwamoto); Protocol/Study Design Committee (Kaoru Yamazaki*, Tatsuhiko Tanizawa, Toshiharu Fujita, and Tosiya Sato); Case Review Committee (Tetsuro Nakamura*, Hiroshi Minaguchi, Kaoru Yamazaki, Tetsuo Inoue, and Toshiharu Fujita); Data Management/Study Execution Committee (Toshiharu Fujita*, Atsuya Ono, Kaoru Yamazaki, Tadataka Mitsuishi, Tetsuro Nakamura, Toshitaka Makino, and Tosiya Sato); Radiological Evaluation Committee (Kazuhiro Kushida*, Akira Kitazawa, Hideaki Kishimoto, Hiroshi Hagino, Hiroshi Katagiri, and Tsuyoshi Oishi); and Ethical/Monitoring Committee (Hiroe Tsubaki*, Akira Itabashi, Kiyoshi Kurokawa, Takahide Kurokawa, Tatsuo Kuroyanagi, and Yoichi Kato).
About this article
Cite this article
Inoue, T., Fujita, T., Kishimoto, H. et al. Randomized controlled study on the prevention of osteoporotic fractures (OF study): a phase IV clinical study of 15-mg menatetrenone capsules. J Bone Miner Metab 27, 66–75 (2009). https://doi.org/10.1007/s00774-008-0008-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-008-0008-8