Abstract
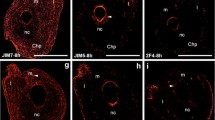
Chalazal endosperm haustorium in Rhinanthus serotinus consists of a single large binucleate cell. It originates from the primary endosperm cell dividing transversely into two unequal cells: a smaller micropylar cell and a larger chalazal cell. The chalazal cell undergoes a single mitotic division, then lengthens significantly during development and functions as a chalazal endosperm haustorium. In this paper, immunofluorescent techniques, rhodamine phalloidin assay, and electron microscopy were used to examine the actin and tubulin cytoskeleton during the development of the chalazal haustorium. During the differentiation stage, numerous longitudinally oriented bundles of microfilaments ran along the axis of transvacuolar strands in haustorium. Microtubules formed intensely fluorescent areas near the nuclear envelope and also formed radial perinuclear microtubule arrays. In the fully differentiated haustorium cell, the actin cytoskeleton formed dense clusters of microfilaments on the chalazal and micropylar poles of the haustorium. Numerous microfilament bundles occurred near wall ingrowths on the chalazal wall. There were numerous clusters of microfilaments and microtubules around the huge lobed polytenic haustorial nuclei. The microfilaments were oriented longitudinally to the long axis of the haustorium cell and surrounded both nuclei. The microtubules formed radial perinuclear systems which were appeared to radiate from the surface of the nuclear envelope. The early stage of degeneration of the chalazal haustorium was accompanied by the degradation of microtubules and disruption of the parallel orientation of microtubules in the chalazal area of the cell. The degree of vacuolization increased, autophagous vacuoles appeared and the number of vesicles decreased.








Similar content being viewed by others
References
Arekal GD (1963) Embryological studies in Canadian representatives of Rhinantheae, Scrophulariaceae. Can J Bot 41:267–302
Bassell G, Singer RH (1997) mRNA and cytoskeletal filaments. Curr Opin Cell Biol 9(1):109–115
Binarová P, Cenková V, Hause B, Kubátová E, Lysák M, Doležel J, Bögre L, Dráber P (2000) Nuclear gamma-tubulin during acentriolar plant mitosis. Plant Cell 12:433–442
Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C (1998) Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant Journal 15:441–447
Bogucka-Glotzer J, Ephrussi A (1996) mRNA localization and the cytoskeleton. Cell & Devlop Biol 7:357–365
Bohdanowicz J, Szczuka E, Świerczyńska J, Sobieska J, Kościńska-Pająk M (2005) The distribution of microtubules during regular and disturbed microsporogenesis and pollen grain development in Gagea lutea (L.) Ker.-Gaw. Acta Biol Cracov Ser Bot 47:89–96
Brodsky VY, Uryvaeva IV (1985) Genome multiplication in growth and development. Biology of polyploid and polytene cells. Cambridge University Press, Cambridge
Brown RC, Lemmon BE, Mulinax JB (1989) Immunofluorescent staining of microtubules in plant tissues: improved embedding and sectioning techniques using polyethylene glycol (PEG) and Steedman’s wax. Bot Acta 102:54–61
Brown R, Lemmon BE, Olsen OA (1994) Endosperm development in barley: microtubule involvement in the morphogenetic pathway. Plant Cell 6:1241–1252
Brown R, Lemmon BE, Olsen OA (1996) Development of endosperm in rice (Oryza sativa L.): cellularization. J Plant Res 109:301–313
Brown R, Lemmon BE, Nguyen H, Olsen OA (1999) Development of endosperm in Arabodopsis thaliana. Sex Plant Reprod 12:32–42
Brown R, Lemmon BE, Nguyen H (2003) Events during first four rounds of mitosis establish three developmental domains in the syncytial endosperm of Arabidopsis thaliana. Protoplasma 222(3–4):167–174
Brown R, Lemmon BE, Nguyen H (2004) Comparative anatomy of the chalazal endosperm cyst in seeds of the Brassicaceae. Bot J Linnean Soc 144:375–395
Bulbert MW, Offler CE, McCurdy DW (1998) Polarized microtubule deposition coincides with wall ingrowth formation in transfer cells of Vicia faba cotyledons. Protoplasma 201:8–16
Czapska-Dziekanowska D (1965) Studies in the mode of reproduction and the differentiation of the endosperm of Plantago atrata var. carpatica. Acta Biol Cracov Ser Bot 8:101–112
Cyr RJ (1994) Microtubules in plant morphogenesis: role of the cortical array. Ann Rev Cell Biol 10:153–180
D’Amato F (1989) Polyploidy in cell differentiation. Caryologia 42:183–211
Davis G (1966) Systematic embryology of the angiosperms. Wiley, New York
Dute RR, Peterson CM (1992) Early endosperm development in ovules of Soyabean, Glycine max (L.) Merr. (Fabaceae). Ann Bot 69:263–271
Enzenberg U (1961) Beiträge zur Karyologie des Endosperms. Öster Bot Z 103:245–285
Erbrich P (1965) Über Endopolyploidie und Kernstrukturen in Endospermhaustorien. Öster Bot Z 112:197–262
Gunning BES, Pate JS (1969) “Transfer cells”. Plant cells with wall ingrowths, specialized in relation to short distance transport of solutes-their occurrence, structure, and development. Protoplasma 68:107–133
Gunning BES, Pate JS (1974) Transfer cells. In: Robards AW (ed) Dynamic aspects of plant ultrastructure. McGraw-Hill, London, pp 441–480
Guo F, Yu L, Watkins S, Han Y (2007) Orientation of microtubules suggests a role in mRNA transportation in fertilized eggs of Chinese pine (Pinus tabulaeformis). Protoplasma 231:239–243
Huang BQ, Russell SD (1994) Fertilization in Nicotiana tabacum: cytoskeletal modifications in the embryo sac during synergid degeneration. Planta 194:200–214
Huang BQ, Ye XL, Yeung EC, Zee SY (1998) Embryology of Cymbidium sinense: the microtubule organization of early embryos. Ann Bot 81:741–750
Joubes J, Chevalier C (2000) Endoreduplication in higher plants. Plant Mol Biol 43(5–6):735–745
Johri BM, Ambegaokar KB (1984) Embryology: then and now. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin, pp 1–52
Kamal A, Goldstein LSB (2000) Conecting vesicle transport to the cytoskeleton. Curr Opin Cell Biol 12(4):503–508
Kandasamy M, Meagher RB (1999) Actin-organelle interaction: association with chloroplasts in Arabidopsis leaf mesophyll cells. Cell Mot Cyt 44:110–118
Ketelaar T, Faivre-Moskalenko C, Esseling JJ, de Ruijter NCA, Grierson CS, Dogterom M, Emons AMC (2002) Positioning of nuclei in Arabidopsis root hairs: an actin-regulated process of tip growth. Plant Cell 14:2941–2955
Kozieradzka-Kiszkurno M, Świerczyńska J, Bohdanowicz J (2011a) Embryogenesis in Sedum acre L.: structural and immunocytochemical aspects of suspensor development. Protoplasma 248:775–784
Kozieradzka-Kiszkurno M, Płachno BJ, Bohdanowicz J (2011b) Are unusual plasmodesmata in the embryo-suspensor restricted to species from the genus Sedum among Crassulaceae? Flora 206:684–690
Kozieradzka-Kiszkurno M, Płachno BJ, Bohdanowicz J (2012) New data about the suspensor of succulent angiosperms: ultrastructure and cytochemical study of the embryo-suspensor of Sempervivum arachnoideum L. and Jovibarba sobolifera (Sims) Opiz. Protoplasma 249:613–624
Liu B, Marc J, Joshi HC, Palevitz BA (1993) A gamma-tubulin-releted protein associated with the microtubule arrays of higher plants in a cell cycle-dependent manner. J Cell Sci 104:1217–1228
Marc J (1997) Microtubule-organizing centres in plants. Trends Plant Sci 6:223–230
Muench DG, Chuang SDX, Franceschi VR, Okita TW (2000) Developing prolamine protein bodies are associate with the cortical cytoskeleton in rice endosperm cells. Planta 211:227–238
Muench DG, Park NI (2006) Messages on the move: the role of the cytoskeleton in mRNA localization and translation in plant cells. Can J Bot 84:572–580
Nagl W (1978) Endopoliploidy and polyteny in differentiation and evolution. North-Holland Publishing Company, Amsterdam
Nagl W (1990) Polyploidy in differentiation and evolution. Int J Cell Clon 8:216–223
Nagl W (1992) The polytenic endosperm haustorium of Rhinanthus minor (Scrophulariaceae): functional ultrastructure. Can J Bot 70:1997–2004
Nasmyth K, Jansen RP (1997) The cytoskeleton in mRNA localization and cell differentiation. Curr Opin Cell Biol 9:396–400
Nguyen H, Brown RC, Lemmon BE (2000) The specialized chalazal endosperm in Arabidopsis thaliana and Lepidium virginicum (Brassicaceae). Protoplasma 212:99–110
Nguyen H, Brown RC, Lemmon BE (2001) Patterns of cytoskeletal organization reflect distinct developmental domains in endosperm of Coronopus didymus (Brassicaceae). Int J Plant Sci 162(1):1–14
Nguyen H, Brown RC, Lemmon BE (2002) Cytoskeletal organization of the endosperm in Coronopus didymus L. (Brassicaceae). Protoplasma 68:107–133
Offler CE, McCurdy DW, Patrick JW, Talbot MJ (2003) Transfer cells: cells specialized for a special purpose. Ann Rev Plant Biol 54:431–454
Olsen OA (2001) Endosperm development: cellularyzation and cell fate specification. Ann Plant Physiol Plant Mol Biol 52:233–267
Olyslaegers G, Verbelen JP (1998) Improved staining of F-actin and colocalization of mitochondria in plant cells. J Microsc 192:73–77
Panteris E, Apostolakos P, Gräf R, Galatis B (2000) Gamma-tubulin colocalizes with microtubule arrays and tubulin paracrystals in dividing vegetative cells of higher plants. Protoplasma 210:179–187
Płachno BJ, Świątek P (2011) Syncytia in plants: cell fusion in endosperm—placental syncytium formation in Utricularia (Lentibulariaceae). Protoplasma 248:425–435
Płachno BJ, Świątek P, Kozieradzka-Kiszkurno M (2011) The F-actin cytoskeleton in syncytia from non-clonal progenitor cells. Protoplasma 248:623–629
Płachno BJ, Świątek P, Sas-Nowosielska H, Kozieradzka-Kiszkurno M (2012) Organisation of the endosperm and endosperm-placenta syncytia in bladderworts (Utricularia, Lentibulariaceae) with emphasis on the microtubule arrangement. Protoplasma. doi:10.1007/s00709-012-0468-5
Raghavan V (1986) Embryogenesis in angiosperms. Cambridge University Press, Cambridge
Sabelli PA, Larkins BA (2009) The development of endosperm in grasses. Plant Phys 149:14–26
Schmid E (1906) Beitraege zur Entwicklungsgeschichte der Scrophulariaceae. Beih Bot Zent 20:175–299
Sonobe S, Shibaoka H (1989) Cortical fine actin filaments in higher plant cells visualized by rhodamine-phalloidin after pretreatment with m-maleimidobenzoyl N-hydroxysuccinimide ester. Protoplasma 148:80–86
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Starr DA, Han M (2003) Anchors away: an actin based mechanism of nuclear positioning. J Cell Sci 116:211–21664
Świerczyńska J, Bohdanowicz J (2003) Microfilament cytoskeleton of endosperm chalazal haustorium of Rhinanthus serotinus (Scrophulariaceae). Acta Biol Cracov Ser Bot 45(1):143–148
Świerczyńska J (2004) The cytoskeleton of endosperm chalazal haustorium of Rhinanthus serotinus (Schönheit) Oborny (Scrophulariaceae). Ph. D. dissertation, University of Gdańsk, Gdańsk (in Polish)
Świerczyńska J, Kozieradzka-Kiszkurno M, Bohdanowicz J (2005) Polyploidization of endosperm chalazal haustorium of Rhinanthus serotinus (Scrophulariaceae). Acta Biol Cracov Ser Bot 47(1):123–128
Świerczyńska J, Bednara J, Bohdanowicz J (2006) The cytoskeleton of endosperm chalazal haustorium of Rhinanthus serotinus. Acta Biol Cracov Ser Bot 48(suppl 1):35
Świerczyńska J, Bohdanowicz J (2007) Visualization of actin cytoskeleton of suspensor basal cell in Alisma plantago-aquatica. In: Book of Abstracts of the 3rd Conference of Polish Society of Experimental Plant Biology, August 26–30, 2007, Warsaw, Poland. p.10
Świerczyńska J, Bohdanowicz J (2010) The immunocytochemical studies of the embryo-suspensor in Gagea lutea (L.) Ker-Gaw. Acta Biol Cracov Ser Bot 52(suppl 1):41
Talbot MJ, Offler CE, McCurdy DW (2002) Transfer cell wall architecture: a contribution towards understanding localized wall deposition. Protoplasma 219:197–209
Tiagi B (1966) Development of the seed and fruit in Rhinanthus major and R. serotinus. Am J Bot 53(7):645–651
Tegeder M, Offler CE, Frommer WB, Patrick JW (2000) Amino acid transporters are localized to transfer cells of developing pea seeds. Plant Physiol 122:319–325
Thompson RD, Hueros G, Becker HA, Maitz M (2001) Development and functions of seed transfer cells. Plant Sci 160:775–783
Traas JA, Doonan JH, Rawlins DJ, Shaw PJ, Watts J, Lloyd CW (1987) An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associated with the dividing nucleus. J Cell Biol 105:387–395
Van Lammeren AAM (1988) Structure and function of the microtubular cytoskeleton during endosperm development in wheat: an immunofluorescence study. Protoplasma 146:18–27
Verchot-Lubicz J, Goldstein RE (2010) Cytoplasmic streaming enables the distribution of molecules and vesicles in large plant cells. Protoplasma 240:99–107
Vijayaraghavan MR, Prabhakar K (1984) The endosperm. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin, pp 319–376
Vitha S, Baluška F, Jasik J, Volkman D, Barlow PW (2000) Stedman’s wax for F-actin visualization. In: Staiger CJ, Baluška F, Volkman D, Barlow PW (eds) Actin: a dynamic framework for multiple plant cell functions. Kluwer Academic Publishers, Dordrecht, pp 619–636
XuHan X, Van Lammeren AAM (1993) Microtubular configurations during the cellularization of coenocytic endosperm in Ranunculus sceleratus L. Sex Plant Reprod 6:127–132
XuHan X, Van Lammeren AAM (1994a) The ultrastructure of seed coat development in Ranunculus sceleratus. Acta Bot Neerl 43:27–37
XuHan X, Van Lammeren AAM (1994b) Microtubular configurations during endosperm development in Phaseolus vulgaris. Can J Bot 72:1489–1495
Zarnack K, Feldbrügge M (2010) Microtubule-dependent mRNA transport in fungi. Eukaryotic Cell 9(7):982–990
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Anne-Catherine Schmit
Rights and permissions
About this article
Cite this article
Świerczyńska, J., Kozieradzka-Kiszkurno, M. & Bohdanowicz, J. Rhinanthus serotinus (Schönheit) Oborny (Scrophulariaceae): immunohistochemical and ultrastructural studies of endosperm chalazal haustorium development. Protoplasma 250, 1369–1380 (2013). https://doi.org/10.1007/s00709-013-0520-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-013-0520-0