Abstract
This article describes a new procedure for multi-element preconcentration of heavy metal ions, specifically of Cr(III), Co(II), Ni(II), Cu(II), Zn(II) and Pb(II) ions. The method is based on dispersive micro solid-phase extraction (DMSPE) of the metal complexes of 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol (5-Br-PADAP) by graphene oxide nanoparticles prior to energy dispersive X-ray fluorescence spectrometric (EDXRF) determination. The effects of pH, amount of graphene oxide, concentration of complexing reagent, sample volume and sorption time were optimized. The influence of commonly encountered other ions was also investigated. Under optimal conditions, the calibration plots cover the 2 to 150 ng mL−1 range for each element. The precision (at a 20 ng mL−1 level for n = 10) is lower than 4.8 %, and the detection limits range is from 0.07 to 0.25 ng mL−1. The DMSPE-EDXRF procedure was successfully applied to the determination of heavy metal ions in water and spiked water samples with recoveries between 94.4 and 103.5 %.

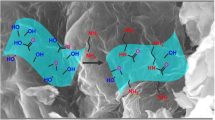
Schematic of the dispersive solid-phase microextraction of metal complexes of 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol (5-Br-PADAP) using graphene oxide nanoparticles, followed by their energy dispersive X-ray fluorescence spectrometric determination.


Similar content being viewed by others
References
Aziz-Zanjani MO, Mehdinia A (2014) A review on procedures for the preparation of coatings for solid phase microextraction. Microchim Acta 181:1169–1190
Zhang B-T, Zheng X, Li H-F, Lin J-M (2013) Application of carbon based nanomaterials in sample preparation: a review. Anal Chim Acta 784:1–17
Silva MM, Arruda MAZ, Krug FJ, Oliveira PV, Queiroz ZF, Gallego M, Valcárcel M (1998) On-line separation and preconcentration of cadmium, lead and nickel in a fullerene (C60) minicolumn coupled to flow injection tungsten coil atomic absorption spectrometry. Anal Chim Acta 368:255–263
Pereira MG, Pereira-Filho ER, Berndt H, Arruda MAZ (2004) Determination of cadmium and lead at low levels by using preconcentration at fullerene coupled to thermospray flame furnace atomic absorption spectrometry. Spectrochim Acta Part B 59:515–521
Chen S, Liu C, Yang M, Lu D, Zhu L, Wang Z (2009) Solid-phase extraction of Cu, Co and Pb on oxidized single-walled carbon nanotubes and their determination by inductively coupled plasma mass spectrometry. J Hazard Mater 170:247–251
Tuzen M, Saygi KO, Soylak M (2008) Solid phase extraction of heavy metal ions in environmental samples on multiwalled carbon nanotubes. J Hazard Mater 152:632–639
Duran A, Tuzen M, Soylak M (2009) Preconcentration of some trace elements via using multiwalled carbon nanotubes as solid phase extraction adsorbent. J Hazard Mater 169:466–471
Liang P, Liu Y, Guo L, Zeng J, Lu H (2004) Multiwalled carbon nanotubes as solid-phase extraction adsorbent for the preconcentration of trace metal ions and their determination by inductively coupled plasma atomic emission spectrometry. J Anal At Spectrom 19:1489–1492
Ozcan SG, Satiroglu N, Soylak M (2010) Column solid phase extraction of iron(III), copper(II), manganese(II) and lead(II) ions food and water samples on multi-walled carbon nanotubes. Food Chem Toxicol 48:2401–2406
Liang P, Liu Y, Guo L (2005) Determination of trace rare earth elements by inductively coupled plasma atomic emission spectrometry after preconcentration with multiwalled carbon nanotubes. Spectrochim Acta Part B 60:125–129
Zawisza B, Skorek R, Stankiewicz G, Sitko R (2012) Carbon nanotubes as a solid sorbent for the preconcentration of Cr, Mn, Fe, Co, Ni, Cu, Zn and Pb prior to wavelength-dispersive X-ray fluorescence spectrometry. Talanta 99:918–923
Wang Y, Ke X, Zhou X, Li J, Ma J (2012) Graphene for separation and preconcentration of trace amounts of cobalt in water samples prior to flame atomic absorption spectrometry. J Saudi Chem Soc. doi:10.1016/j.jscs.2012.10.004
Wang Y, Gao S, Zang X, Li J, Ma J (2012) Graphene-based solid-phase extraction combined with flame atomic absorption spectrometry for a sensitive determination of trace amounts of lead in environmental water and vegetable samples. Anal Chim Acta 716:112–118
Chang Q, Song S, Wang Y, Li J, Ma J (2012) Application of graphene as a sorbent for preconcentration and determination of trace amounts of chromium(III) in water samples by flame atomic absorption spectrometry. Anal Methods 4:1110–1116
Zawisza B, Sitko R, Malicka E, Talik E (2013) Graphene oxide as a solid sorbent for the preconcentration of cobalt, nickel, copper, zinc and lead prior to determination by energy-dispersive X-ray fluorescence spectrometry. Anal Methods 5:6425–6430
Pourjavid MR, Sehat AA, Arabieh M, Yousefi SR, Hosseini MH, Rezaee M (2014) Column solid phase extraction and flame atomic absorption spectrometric determination of manganese(II) and iron(III) ions in water, food and biological samples using 3-(1-methyl-1 H-pyrrol-2-yl)-1 H-pyrazole-5-carboxylic acid on synthesized graphene oxide. Mat Sci Eng C 35:370–378
Liang X, Liu S, Wang S, Guo Y, Jiang S (2014) Carbon-based sorbents: carbon nanotubes. J Chromatogr A 1357:53–67
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multi residue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86:412–431
Sitko R, Zawisza B, Malicka E (2009) Energy-dispersive X-ray fluorescence spectrometer for analysis of conventional and micro-samples: preliminary assessment. Spectrochim Acta Part B 64:436–441
ISO 11885. http://www.iso.org/iso/catalogue_detail.htm?csnumber=36250. Accessed 24 April 2015
Sitko R, Zawisza B, Malicka E (2013) Graphene as a new sorbent in analytical chemistry. Trends Anal Chem 51:33–43
Marczenko Z, Balcerzak M (2000) Separation, preconcentration and spectrophotometry in inorganic analysis. Elsevier, Amsterdam
Sitko R (2009) Quantitative X-ray fluorescence analysis of samples of less than ‘infinite thickness’: difficulties and possibilities. Spectrochim Acta Part B 64:1161–1172
Margui E, Zawisza B, Skorek R, Theato T, Queralt I, Hidalgo M, Sitko R (2013) Analytical possibilities of different X-ray fluorescence systems for determination of trace elements in aqueous samples pre-concentrated with carbon nanotubes. Spectrochim Acta Part B 88:192–197
Kocot K, Leardi R, Walczak B, Sitko R (2015) Determination and speciation of trace and ultratrace selenium ions by energy-dispersive X-ray fluorescence spectrometry using graphene as solid adsorbent in dispersive micro-solid phase extraction. Talanta 134:360–365
Skorek R, Zawisza B, Margui E, Queralt I, Sitko R (2013) Dispersive micro solid-phase extraction using multiwalled carbon nanotubes for simultaneous determination of trace metal ions by energy-dispersive X-ray fluorescence spectrometry. Appl Spectrosc 67:204–209
Zawisza B, Skorek R, Stankiewicz G, Sitko R (2012) Carbon nanotubes as a solid sorbent for the preconcentration of Cr, Mn, Fe, Co, Ni, Cu, Zn and Pb prior to wavelength-dispersive X-ray fluorescence spectrometry. Talanta 99:918–923
Kocot K, Sitko R (2014) Trace and ultratrace determination of heavy metal ions by energy dispersive X-ray fluorescence spectrometry using graphene as solid sorbent in dispersive micro solid-phase extraction. Spectrrochim Acta Part B 94–95:7–13
Faraji M, Yamini Y, Saleh A, Rezaee M, Ghambarian M, Hassani R (2010) A nanoparticle-based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal Chim Acta 659:172–177
Jamshidi M, Ghaedi M, Mortazavi K, Nejati Biareh M, Soylak M (2011) Determination of some metal ions by flame-AAS after their preconcentration using sodium dodecyl sulfate coated alumina modified with 2-hydroxy-(3-((1-H-indol 3-yle)phenyl) methyl) 1-H-indol (2-HIYPMI). Food Chem Toxicol 49:1229–1234
Dados A, Paparizou E, Eleftheriou P, Papastephanou C, Stalikas CD (2014) Nanometer-sized ceria-coated silica–iron oxide for the reagentless microextraction/preconcentration of heavy metals in environmental and biological samples followed by slurry introduction to ICP-OES. Talanta 121:127–135
Mortada WI, Kenawy IMM, Abdelghany AM, Ismail AM, Donia AF, Nabieh KA (2015) Determination of Cu2+, Zn2+ and Pb2+ in biological and food samples by FAAS after preconcentration with hydroxyapatite nanorods originated from eggshell. Mater Sci Eng C 52:288–296
Vieiraa EG, Soares IV, Dias Filho NL, da Silva NC, Perujo SD, Bastos AC, Garcia EF, Ferreira TT, Fracetob LF, Rosa AH (2012) Study on soluble heavy metals with preconcentration by using a new modified oligosilsesquioxane sorbent. J Hazard Mater 237–238:215–222
Karadaş C, Turhan O, Kara D (2013) Synthesis and application of a new functionalized resin for use in an on-line, solid phase extraction system for the determination of trace elements in waters and reference cereal materials by flame atomic absorption spectrometry. Food Chem 141:655–661
He Q, Chang X, Huang X, Hu Z (2008) Determination of trace elements in food samples by ICP-AES after preconcentration with p-toluenesulfonylamide immobilized on silica gel and nanometer SiO2. Microchim Acta 160:147–152
ZA ALO, Habila M, Yilmaz E, Soylak M (2012) Solid phase extraction of Cd(II), Pb(II), Zn(II) and Ni(II) from food samples using multiwalled carbon nanotubes impregnated with 4-(2-thiazolylazo)resorcinol. Microchim Acta 177:397–403
Taghizadeh M, Asgharinezhad AA, Pooladi M, Barzin M, Abbaszadeh A, Tadjarodi A (2013) A novel magnetic metal organic framework nanocomposite for extraction and preconcentration of heavy metal ions, and its optimization via experimental design methodology. Microchim Acta 180:1073–1084
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pytlakowska, K. Dispersive micro solid-phase extraction of heavy metals as their complexes with 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol using graphene oxide nanoparticles. Microchim Acta 183, 91–99 (2016). https://doi.org/10.1007/s00604-015-1596-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1596-3