Abstract
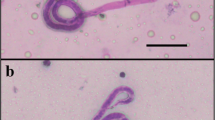
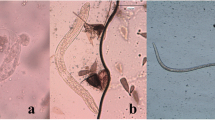
Exsheathment and midgut invasion of nocturnally subperiodic Brugia malayi microfilariae were analyzed using light and scanning electron microscopy in a refractory vector, Aedes aegypti (Thailand strain). Results showed that exsheathed microfilariae represented only approximately 1 % of the total microfilaria midguts dissected at 5-min post-infected blood meal (PIBM). The percentage of exsheathed microfilariae found in midguts progressively increased to about 20, 60, 80, 90, and 100 % at 1-, 2–5-, 6–12-, 18–36-, and 48-h PIBM, respectively. Importantly, all the microfilariae penetrating the mosquito midguts were exsheathed. Midgut invasion by the exsheathed microfilariae was observed between 2- and 48-h PIBM. SEM analysis revealed sheathed microfilariae surrounded by small particles and maceration of the microfilarial sheath in the midguts, suggesting that the midguts of the refractory mosquitoes might have protein(s) and/or enzyme(s) and/or factor(s) that induce and/or accelerate exsheathment. The microfilariae penetrated the internal face of the peritrophic matrix (PM) by their anterior part and then the midgut epithelium, before entering the hemocoel suggesting that PM was not a barrier against the microfilariae migrating towards the midgut. Melanized microfilariae were discovered in the hemocoel examined at 96-h PIBM suggesting that the refractory mosquitoes used melanization reactions against this parasite. This study provided evidence that A. aegypti (Thailand strain) has refractory mechanisms against B. malayi in both midgut and hemocoel.





Similar content being viewed by others
References
Agudelo-Silva F, Spielman A (1985) Penetration of mosquito midgut wall by sheathed microfilariae. J Invertebr Pathol 45:117–119
Bangs MJ, Ash LR, Barr AR (1995) Susceptibility of various mosquitoes of California to subperiodic Brugia malayi. Acta Trop 59:323–332
Barillas-Mury C, Wells MA (1993) Cloning and sequencing of the blood meal-induced late trypsin gene from the mosquito Aedes aegypti and characterization of the upstream regulatory region. Insect Mol Biol 2:7–12
Bian G, Raikhel AS, Zhu J (2008) Characterization of a juvenile hormone-regulated chymotrypsin-like serine protease gene in Aedes aegypti mosquito. Insect Biochem Mol Biol 38:190–200
Billingsley PF, Rudin W (1992) The role of the mosquito peritrophic membrane in bloodmeal digestion and infectivity of Plasmodium species. J Parasitol 78:430–440
Chaithong U (1976) Study on Brugia pahangi infection in Aedes albopictus, Aedes aegypti and Aedes togoi. Thesis (M.Sc. (Tropical Medicine)--Mahidol University
Chang MS, Chan KL, Ho BC (1991) Comparative transmission potential of three Mansonia mosquitoes (Diptera: Culicidae) for filariasis in Sarawak, Malaysia. Bull Entomol Res 81:437–444
Chen CC, Shin CM (1988) Exsheathment of microfilariae of Brugia pahangi in the susceptible and refractory strains of Aedes aegypti. Ann Trop Med Parasitol 82:201–206
Chomcharn Y, Surathin K, Bunnag D, Sucharit S, Harinasuta T (1980) Effect of a single dose of primaquine on a Thai strain of Plasmodium falciparum. Southeast Asian J Trop Med Public Health 11:408–412
Choochote W, Chaithong U, Somboon P, Pakdicharoen A, Tookyang B, Likitvong K, Siriprasert V, Sukontasan K, Thitasut P (1991) Small laboratory animal model for nocturnally subperiodic Brugia malayi (Narathiwat province, southern Thailand strain). J Trop Med Parasitol 14:51–58
Choochote W, Sukhavat K, Somboon P, Khamboonruang C, Maleewong W, Suwanpanit P (1986) The susceptibility of small laboratory animals to nocturnally superiodic Brugia malayi in Thailand. J Parasitol Trop Med Assoc Thailand 9:35–37
Christensen BM, Sutherland DR (1984) Brugia pahangi: exsheathment and midgut penetration in Aedes aegypti. Trans Amer Microscop Soc 4:423–433
Christensen BM, Li J, Chen CC, Nappi A (2005) Melanization immune response in mosquito vectors. Trends Parasitol 21:192–199
Denham DA, McGreevy PB (1977) Brugian filariasis: epidemiological and experimental studies. Adv Parasitol 15:243–309
Devaney E, Howells RE (1979) The exsheathment of Brugia pahangi microfilariae under controlled conditions in vitro. Ann Trop Med Parasitol 73:227–333
Edwards MJ, Moskalyk LA, Donelly-Doman M, Vlaskova M, Noriega FG, Walker VK, Jacobs-Lorena M (2000) Characterization of a carboxypeptidase A gene from the mosquito, Aedes aegypti. Insect Mol Biol 9:33–38
Erickson SM, Xi Z, Mayhew GF, Ramirez JL, Aliota MT, Christensen BM, Dimopoulos G (2009) Mosquito infection responses to developing filarial worms. PLoS Negl Trop Dis 3:e529
Esslinger JH (1962) Behavior of microfilaria of Brugia pahangi in Anopheles quadrimaculatus. Am J Trop Med Hyg 11:749–758
Ewert A (1965) Comparative migration of microfilariae and development of Brugia pahangi in various mosquitoes. Am J Trop Med Hyg 14:254–259
Felix CR, Betschart B, Billingsley PF, Freyvogel TA (1991) Post -feeding induction of trypsin in the midgut of Aedes aegypti is separable into two cellular phases. Insect Bioch 21:197–203
Guptavanij P, Harinasuta C, Vutikes S, Deesin T, Surathin K (1978) The vectors of Brugia malayi in southern Thailand. Southeast Asian J Trop Med Public Health 9:543–548
Isoe J, Zamora J, Miesfeld RL (2009a) Molecular analysis of the Aedes aegypti carboxypeptidase gene family. Insect Biochem Mol Biol 39:68–73
Isoe J, Rascon AA Jr, Kunz S, Miesfeld RL (2009b) Molecular genetic analysis of midgut serine proteases in Aedes aegypti mosquitoes. Insect Biochem Mol Biol 39:903–912
Iyengar MOT (1936) Entry of Wlaria larvae into the body cavity of the mosquito. Parasitology 28:190–195
Jariyapan N, Saeung A, Intakhan N, Chanmol W, Sor-Suwan S, Phattanawiboon B, Taai K, Choochote W (2013) Peritrophic matrix formation and Brugia malayi microfilaria invasion of the midgut of a susceptible vector, Ochlerotatus togoi (Diptera: Culicidae). Parasitol Res 112:2431–2440
Jiang Q, Hall M, Noriega FG, Wells M (1997) cDNA cloning and pattern of expression of an adult, female specific chymotrypsin from Aedes aegypti midgut. Insect Biochem Mol Biol 27:283–289
Kalhok SE, Tabak LM, Prosser DE, Brook W, Downe AE, White BN (1993) Isolation, sequencing and characterization of two cDNA clones coding for trypsin-like enzymes from the midgut of Aedes aegypti. Insect Mol Biol 2:71–79
Kershaw WE, Edeson JF, Johnson M (1961) The intake of Brugia malayi by different strains of Aedes aegypti. Ann Soc Belg Med Trop 41:281–282
Kumar NP, Sabesan S, Panicker KN (1998) Susceptibility status of Mansonia annulifera to Brugia malayi parasites in Cherthala, Alappuzha district, Kerala. Indian J Exp Biol 36:829–831
Lek-Uthai U, Tomoen W (2005) Susceptibility of Mansonia uniformis to Brugia malayi microfilariae from infected domestic cat. Southeast Asian J Trop Med Public Health 36:434–441
Luo H, Qu FY (1990) Experimental infection index of Anopheles sinensis and melanization of periodic Brugia malayi. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 8:260–263
Magalhaes T, Oliveira IF, Melo-Santos MAV, Oliveira CMF, Lima CA, Ayres CFJ (2008) Expression of defensin, cecropin, and transferrin in Aedes aegypti (Diptera: Culicidae) infected with Wuchereria bancrofti (Spirurida: Onchocercidae), and the abnormal development of nematodes in the mosquito. Exp Parasitol 120:364–371
Michalski ML, Erickson SM, Bartholomay LC, Christensen BM (2010) Midgut barrier imparts selective resistance to filarial worm infection in Culex pipiens pipiens. PLoS Negl Trop Dis 4:e875
Nayar JK, Knight JW (1995) Comparison of migration and encapsulation of Brugia malayi microfilariae from the midgut to the hemocoel between Anopheles quadrimaculatus and Aedes aegypti. J Invertebr Pathol 65:295–299
Nelson RL (1964) Parity in winter populations of Culex tarsalis Coquillett in Kern County, California. Amer J Hyg 80:242–253
Noriega FG, Edgar KA, Bechet R, Wells MA (2002) Midgut exopeptidase activities in Aedes aegypti are induced by blood feeding. J Insect Physiol 48:205–212
O’Connor FW, Beatty H (1936) The early migrations of Wuchereria bancrofti in Culex fatigans. Trans R Soc Trop Med Hyg 30:125–127
Oda T, Wada Y (1980) Exsheathment and migration of microfilariae of Brugia malayi (Che-ju Strain) in mosquitoes. Trop Med 22:27–33
Owen RR (1979) Non-development of Brugia pahangi in a refractory mosquito, Aedes malayansis. Ann Trop Med Parasit 73:193–195
Perrone JB, Spielman A (1986) Microfilarial perforation of the midgut of a mosquito. J Parasitol 72:723–727
Pothikasikorn J, Bangs MJ, Boonplueang R, Chareonviriyaphap T (2008) Susceptibility of various mosquitoes of Thailand to nocturnal subperiodic Wuchereria bancrofti. J Vector Ecol 33:313–320
Rao UR, Vickery AC, Kwa BH, Nayar JK (1992) Brugia malayi: ivermectin inhibits the exsheathment of microfilariae. Am J Trop Med Hyg 46:183–188
Saboia-Vahia L, Borges-Veloso A, Cuervo P, Junqueira M, Mesquita-Rodrigues C, Britto C, Domont GB, De Jesus JB (2012) Protein expression in the midgut of sugar-fed Aedes albopictus females. Parasit Vectors 5:290
Saeung A, Choochote W (2013) Development of a facile system for mass production of Brugia malayi in a small-space laboratory. Parasitol Res 112:3259–3265
Saeung A, Hempolchom C, Baimai V, Thongsahuan S, Taai K, Jariyapan N, Chaithong U, Choochote W (2013) Susceptibility of eight species members in the Anopheles hyrcanus group to nocturnally subperiodic Brugia malayi. Parasit Vectors 6:5
Sakamoto M, Ohta M, Suzuki A, Takase H, Yoshizawa Y, Kitami M, Sato R (2011) Localization of the serine protease homolog BmSPH-1 in nodules of E. coli-injected Bombyx mori larvae and functional analysis of its role in nodule melanization. Dev Comp Immunol 35:611–619
Santos JN, Lanfredi RM, Pimenta PF (2006) The invasion of the midgut of the mosquito Culex (Culex) quinquefasciatus Say, 1823 by the helminth Litomosoides chagasfilhoi Moraes Neto, Lanfredi and De Souza, 1997. J Invertebr Pathol 93:1–10
Schacher JF (1962) Morphology of the microfilaria of Brugia pahangi and of the larval stages in the mosquito. J Parasitol 48:679–692
Tchankouo-Nguetcheu S, Khun H, Pincet L, Roux P, Bahut M, Huerre M, Guette C, Choumet V (2010) Differential protein modulation in midguts of Aedes aegypti infected with Chikungunya and dengue 2 viruses. PLoS One 5:e13149
Thomas P, Kenny N, Eyles D, Moreira LA, O’Neill SL, Asgari S (2011) Infection withthe wMel and wMelPop strains of Wolbachia leads to higher levels of melanization in the hemolymph of Drosophila melanogaster, Drosophila simulans and Aedes aegypti. Dev Comp Immunol 35:360–365
Tokura A, Fu GS, Sakamoto M, Endo H, Tanaka S, Kikuta S, Tabunoki H, Sato R (2014) Factors functioning in nodule melanization of insects and their mechanisms of accumulation in nodules. J Insect Physiol 60:40–49
Trpis M (1981) Susceptibility of the autogenous group of the Aedes scutellaris complex of mosquitoes to infection with Brugia malayi and Brugia pahangi. Tropenmed Parasitol 32:184–188
Townson H, Chaithong U (1991) Mosquito host influences on development of filariae. Ann Trop Med Parsitol 85:149–163
Wada Y (2011) Vector mosquitoes of filariasis in Japan. Trop Med Health 39:39–45
Weinstein PP (1963) Development in vitro of the microfilariae of Wuchereria bancrofti and of Litomosoides carinii as far as the sausage form. Trans R Soc Trop Med Hyg 57:236
Yamamoto H, Ogura N, Kobayashi M, Chigusa Y (1983) Studies on filariasis II: exsheathment of the microfilariae of Brugia pahangi in Armigeres subalbatus. Jpn J Parasitol 32:287–292
Zou Z, Shin SW, Alvarez KS, Kokoza V, Raikhel AS (2010) Distinct melanization pathways in the mosquito Aedes aegypti. Immunity 32:41–53
Acknowledgments
This work was financially supported by the Thailand Research Fund (TRF Senior Research Scholar: RTA5480006 to WC, subproject to NJ), and the Faculty of Medicine Endowment Fund, Chiang Mai University.
Author information
Authors and Affiliations
Corresponding author
Additional information
This publication is dedicated to the memory of our esteemed colleague, Professor Wej Choochote
Rights and permissions
About this article
Cite this article
Intakhan, N., Jariyapan, N., Sor-suwan, S. et al. Exsheathment and midgut invasion of nocturnally subperiodic Brugia malayi microfilariae in a refractory vector, Aedes aegypti (Thailand strain). Parasitol Res 113, 4141–4149 (2014). https://doi.org/10.1007/s00436-014-4086-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4086-3