Abstract
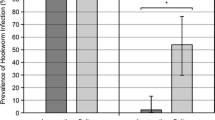
Understanding the fundamental factors influencing the epidemiology of wildlife disease is essential to determining the impact of disease on individual health and population dynamics. The host–pathogen–environment relationship of the endangered Australian sea lion (Neophoca cinerea) and the parasitic hookworm, Uncinaria sanguinis, was investigated in neonatal pups during summer and winter breeding seasons at two biogeographically disparate colonies in South Australia. The endemic occurrence of hookworm infection in Australian sea lion pups at these sites was 100 % and post-parturient transmammary transmission is likely the predominant route of hookworm infection for pups. The prepatent period for U. sanguinis in Australian sea lion pups was determined to be 11–14 days and the duration of infection approximately 2–3 months. The mean hookworm infection intensity in pups found dead was 2138 ± 552 (n = 86), but a significant relationship between infection intensity and faecal egg count was not identified; infection intensity in live pups could not be estimated from faecal samples. Fluctuations in infection intensity corresponded to oscillations in the magnitude of colony pup mortality, that is, higher infection intensity was significantly associated with higher colony pup mortality and reduced pup body condition. The dynamic interaction between colony, season, and host behaviour is hypothesised to modulate hookworm infection intensity in this species. This study provides a new perspective to understanding the dynamics of otariid hookworm infection and provides evidence that U. sanguinis is a significant agent of disease in Australian sea lion pups and could play a role in population regulation in this species.


Similar content being viewed by others
References
Albon SD, Stien A, Irvine RJ, Langvatn R, Ropstad E, Halvorsen O (2002) The role of parasites in the dynamics of a reindeer population. Proc R Soc Lond B 269:1625–1632. doi:10.1098/rspb.2002.2064
Anderson RM, Schad GA (1985) Hookworm burdens and faecal egg counts: an analysis of the biological basis of variation. Trans R Soc Trop Med Hyg 79:812–825. doi:10.1016/0035-9203(85)90128-2
Baylis AMM, Hamer DJ, Nichols PD (2009) Assessing the use of milk fatty acids to infer the diet of the Australian sea lion (Neophoca cinerea). Wildl Res 36:169–176. doi:10.1071/WR08046
Baylis HA (1933) A new species of the nematode genus Uncinaria from a sea-lion, with some observations on related species. Parasitology 25:308–316. doi:10.1017/S0031182000019508
Baylis HA (1947) A redescription of Uncinaria lucasi Stiles, a hookworm of seals. Parasitology 38:160–162. doi:10.1017/S0031182000023118
Berón-Vera B, Crespo EA, Raga JA, Pedraza SN (2004) Uncinaria hamiltoni (Nematoda: Ancylostomatidae) in South American sea lions, Otaria flavescens, from Northern Patagonia, Argentina. J Parasitol 90:860–863. doi:10.1645/GE-182R
Burke TM, Roberson EL (1985) Prenatal and lactational transmission of Toxocara canis and Ancylostoma caninum: experimental infection of the bitch at midpregnancy and at parturition. Int J Parasitol 15:485–490. doi:10.1016/0020-7519(85)90041-4
Campbell RA, Gales NJ, Lento GM, Baker CS (2008) Islands in the sea: extreme female natal site fidelity in the Australian sea lion, Neophoca cinerea. Biol Lett 4:139–142. doi:10.1098/rsbl.2007.0487
Castinel A, Duignan PJ, Lyons ET, Pomroy WE, Gibbs NJ, Lopez-Villalobos N, Chilvers BL, Wilkinson IS (2007) Epidemiology of hookworm (Uncinaria spp.) infection in New Zealand (Hooker’s) sea lion (Phocarctos hookeri) pups on Enderby Island, Auckland Islands (New Zealand) during the breeding seasons from 1999/2000 to 2004/2005. Parasitol Res 101:53–62. doi:10.1007/s00436-006-0453-z
Chilvers BL, Duignan PJ, Robertson BC, Castinel A, Wilkinson IS (2009) Effects of hookworms (Uncinaria sp.) on the early growth and survival of New Zealand sea lion (Phocarctos hookeri) pups. Polar Biol 32:295–302. doi:10.1007/s00300-008-0559-0
Christensen NØ, Nansen P, Fagbemi BO, Monrad J (1987) Heterologous antagonistic and synergistic interactions between helminths and between helminths and protozoans in concurrent experimental infection of mammalian hosts. Parasitol Res 73:387–410. doi:10.1007/BF00538196
Coyne MJ, Smith G (1992) The mortality and fecundity of Haemonchus contortus in parasite-naive and parasite-exposed sheep following single experimental infections. Int J Parasitol 22:315–325. doi:10.1016/S0020-7519(05)80009-8
Davey D, Manickam N, Simms BT, Harrison LM, Vermeire JJ, Cappello M (2013) Frequency and intensity of exposure mediate resistance to experimental infection with the hookworm, Ancylostoma ceylanicum. Exp Parasitol 133:243–249. doi:10.1016/j.exppara.2012.11.010
DeLong RL, Orr AJ, Jenkinson RS, Lyons ET (2009) Treatment of northern fur seal (Callorhinus ursinus) pups with ivermectin reduces hookworm-induced mortality. Mar Mamm Sci 25:944–948. doi:10.1111/j.1748-7692.2008.00274.x
Gales NJ, Cheal AJ, Pobar GJ, Williamson P (1992) Breeding biology and movements of Australian sea-lions, Neophoca cinerea, off the west coast of Western Australia. Wildl Res 19:405–416. doi:10.1071/WR9920405
Gales NJ, Shaughnessy PD, Dennis TE (1994) Distribution, abundance and breeding cycle of the Australian sea lion Neophoca cinerea (Mammalia: Pinnipedia). J Zool 234:353–370. doi:10.1111/j.1469-7998.1994.tb04853.x
George-Nascimento M, Lima M, Ortiz E (1992) A case of parasite-mediated competition? Phenotypic differentiation among hookworms Uncinaria sp. (Nematoda: Ancylostomatidae) in sympatric and allopatric populations of South American sea lions Otaria byronia, and fur seals Arctocephalus australis (Carnivora: Otariidae). Mar Biol 112:527–533. doi:10.1007/BF00346169
Goldsworthy SD, Gales NJ (2008) Neophoca cinerea. In: IUCN red list of threatened species. Version 2013.2. http://www.iucnredlist.org/details/14549. Accessed 1 May 2014
Goldsworthy SD, McKenzie J, Shaughnessy PD, McIntosh RR, Page B, Campbell RA (2009) An update of the report: understanding the impediments to the growth of Australian sea lion populations. SARDI Publication Number F2008/00847-1. SARDI Research Report series No. 356, Adelaide, Australia
Goldsworthy SD, Page B, Lowther AD, Shaughnessy PD (2012) Maintaining the monitoring of pup production at key Australian sea lion colonies in South Australia (2010/11). SARDI Publication Number F2010/000665-2. SARDI Research Report series no. 601, Adelaide, Australia
Goldsworthy SD, Lowther AD, Shaughnessy PD (2013) Maintaining the monitoring of pup production at key Australian sea lion colonies in South Australia (2011/12). SARDI Publication Number F2010/000665-3. SARDI Research Report series no. 739, Adelaide, Australia
Hamer GL, Anderson TK, Berry GE, Makohon-Moore AP, Crafton JC, Brawn JD, Dolinski AC, Krebs BL, Ruiz MO, Muzzall PM, Goldberg TL, Walker ED (2013) Prevalence of filarioid nematodes and trypanosomes in American robins and house sparrows, Chicago USA. Int J Parasitol Parasites Wildl 2:42–49. doi:10.1016/j.ijppaw.2012.11.005
Haynes BT, Marcus AD, Šlapeta J, Gray R (2013) Hookworms (Uncinaria sp.) do not segregate according to the restriction of the host, the Australian sea lion (Neophoca cinerea). Honours Dissertation, The University of Sydney
Hernández-Orts JS, Montero FE, Juan-García A, García NA, Crespo EA, Raga JA, Aznar FJ (2012) Intestinal helminth fauna of the South American sea lion Otaria flavescens and fur seal Arctocephalus australis from northern Patagonia, Argentina. J Helminthol 1:1–12. doi:10.1017/S0022149X12000454
Higgins LV, Tedman RA (1990) Effect of attacks by male Australian sea lions, Neophoca cinerea, on mortality of pups. J Mammal 71:617–619. doi:10.2307/1381802
Higgins LV (1993) The nonannual, nonseasonal breeding cycle of the Australian sea lion, Neophoca cinerea. J Mammal 74:270–274. doi:10.2307/1382381
Higgins LV, Gass L (1993) Birth to weaning: parturition, duration of lactation, and attendance cycles of Australian sea lions (Neophoca cinerea). Can J Zool 71:2047–2055. doi:10.1139/z93-290
Hudson PJ, Dobson AP, Newborn D (1998) Prevention of population cycles by parasite removal. Science 282:2256–2258. doi:10.1126/science.282.5397.2256
Huffman MA, Gotoh S, Turner LA, Hamai M, Yoshida K (1997) Seasonal trends in intestinal nematode infection and medicinal plant use among chimpanzees in the Mahale Mountains, Tanzania. Primates 38:111–125. doi:10.1007/BF02382002
Irvine RJ (2006) Parasites and the dynamics of wild mammal populations. Anim Sci 82:775–781. doi:10.1017/ASC2006106
Katz H, Morgades D, Castro-Ramos M (2012) Pathological and parasitological findings in South American fur seal pups (Arctocephalus australis) in Uruguay. ISRN Zool. doi:10.5402/2012/586079
Keyes MC (1965) Pathology of the northern fur seal. J Am Vet Med Assoc 147:1090–1095
Kretzmann MB, Costa DP, Higgins LV, Needham DJ (1991) Milk composition of Australian sea lions, Neophoca cinerea: variability in lipid content. Can J Zool 69:2556–2561. doi:10.1139/z91-360
Krupp IM (1961) Effects of crowding and of superinfection on habitat selection and egg production in Ancylostoma caninum. J Parasitol 47:957–961. doi:10.2307/3275030
Lowther AD, Goldsworthy SD (2011) Maternal strategies of the Australian sea lion (Neophoca cinerea) at Dangerous Reef, South Australia. Aust J Zool 59:54–62. doi:10.1071/ZO11025
Lowther AD, Harcourt RG, Goldsworthy SD, Stow A (2012) Population structure of adult female Australian sea lions is driven by fine-scale foraging site fidelity. Anim Behav 83:691–701. doi:10.1016/j.anbehav.2011.12.015
Lyons ET, Keyes MC (1984) Further indication of viability of larvae of the hookworm (Uncinaria lucasi) for several years in tissues of northern fur seals (Callorhinus ursinus). J Parasitol 70:459–460. doi:10.2307/3281589
Lyons ET (1994) Vertical transmission of nematodes: emphasis on Uncinaria lucasi in northern fur seals and Strongyloides westeri in equids. J Helminthol Soc Wash 61:169–178
Lyons ET, DeLong RL, Melin SR, Tolliver SC (1997) Uncinariasis in northern fur seal and California sea lion pups from California. J Wildl Dis 33:848–852. doi:10.7589/0090-3558-33.4.848
Lyons ET, DeLong RL, Gulland FMD, Melin SR, Tolliver SC, Spraker TR (2000a) Comparative biology of Uncinaria spp. in the California sea lion (Zalophus californianus) and the northern fur seal (Callorhinus ursinus) in California. J Parasitol 86:1348–1352. doi:10.2307/3285025
Lyons ET, Spraker TR, Olson KD, Tolliver SC, Bair HD (2000b) Prevalence of hookworms (Uncinaria lucasi Stiles) in northern fur seal (Callorhinus ursinus Linnaeus) pups on St. Paul Island, Alaska, U.S.A.: 1986–1999. Comp Parasitol 67:218–223
Lyons ET, Melin SR, DeLong RL, Orr AJ, Gulland FMD, Tolliver SC (2001) Current prevalence of adult Uncinaria spp. in northern fur seal (Callorhinus ursinus) and California sea lion (Zalophus californianus) pups on San Miguel Island, California, with notes on the biology of these hookworms. Vet Parasitol 97:309–318. doi:10.1016/S0304-4017(01)00418-6
Lyons ET, DeLong RL, Spraker TR, Melin SR, Tolliver SC (2003) Observations in 2001 on hookworms (Uncinaria spp.) in otariid pinnipeds. Parasitol Res 89:503–505. doi:10.1007/s00436-002-0784-3
Lyons ET, Delong RL, Spraker TR, Melin SR, Laake JL, Tolliver SC (2005) Seasonal prevalence and intensity of hookworms (Uncinaria spp.) in California sea lion (Zalophus californianus) pups born in 2002 on San Miguel Island, California. Parasitol Res 96:127–132. doi:10.1007/s00436-005-1335-5
Lyons ET, Spraker TR, DeLong RL, Ionita M, Melin SR, Nadler SA, Tolliver SC (2011) Review of research on hookworms (Uncinaria lucasi Stiles, 1901) in northern fur seals (Callorhinus ursinus Linnaeus, 1758). Parasitol Res 109:257–265. doi:10.1007/s00436-011-2420-6
Lyons ET, Kuzmina TA, Tolliver SC, Spraker TR (2012) Update on the prevalence of the hookworm, Uncinaria lucasi, in northern fur seals (Callorhinus ursinus) on St. Paul Island, Alaska, 2011. Parasitol Res 111:1397–1400. doi:10.1007/s00436-012-2881-2
Marcus AD, Higgins DP, Šlapeta J, Gray R (2014) Uncinaria sanguinis sp. n. (Nematoda: Ancylostomatidae) from the endangered Australian sea lion, Neophoca cinerea (Carnivora: Otariidae). Folia Parasitol. doi:10.14411/fp.2014.037
McIntosh RR, Goldsworthy SD, Shaughnessy PD, Kennedy CW, Burch P (2012) Estimating pup production in a mammal with an extended and aseasonal breeding season, the Australian sea lion (Neophoca cinerea). Wildl Res 39:137–148. doi:10.1071/WR10222
McIntosh RR, Arthur AD, Dennis TE, Berris M, Goldsworthy SD, Shaughnessy PD, Teixeira CEP (2013) Survival estimates for the Australian sea lion: Negative correlation of sea surface temperature with cohort survival to weaning. Mar Mamm Sci 29:84–108. doi:10.1111/j.1748-7692.2011.00558.x
McIntosh RR, Kennedy CW (2013) Morphology, sex ratio and cause of death in Australian sea lion (Neophoca cinerea) pups. Aust Mammal 35:93–100. doi:10.1071/AM12037
Mizuno A (1997) Ecological study on the hookworm, Uncinaria lucasi, of northern fur seal, Callorhynus ursinus, in Bering Island, Russia. Jpn J Vet Res 45:109–110
Nadler SA, Lyons ET, Pagan C, Hyman D, Lewis EE, Beckmen K, Bell CM, Castinel A, DeLong RL, Duignan PJ, Farinpour C, Huntington KB, Kuiken T, Morgades D, Naem S, Norman R, Parker C, Ramos PW, Spraker TR, Berón-Vera B (2013) Molecular systematics of pinniped hookworms (Nematoda: Uncinaria): species delimitation, host associations and host-induced morphometric variation. Int J Parasitol 43:1119–1132. doi:10.1016/j.ijpara.2013.08.006
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R 2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. doi:10.1111/j.2041-210x.2012.00261.x
Olsen OW (1958) Hookworms, Uncinaria lucasi Stiles, 1901, in fur seals, Callorhinus ursinus (Linn.), on the Pribilof Islands. In: Trefethen JB (ed) Transactions of the twenty-third North American wildlife conference. St. Louis, Missouri, 3–5 March 1958. pp 152–175
Olsen OW, Lyons ET (1965) Life cycle of Uncinaria lucasi Stiles, 1901 (Nematoda: Ancylostomatidae) of fur seals, Callorhinus ursinus Linn., on the Pribilof Islands, Alaska. J Parasitol 51:689–700. doi:10.2307/3276141
Poulin R (1997) Population abundance and sex ratio in dioecious helminth parasites. Oecologia 111:375–380. doi:10.1007/s004420050248
Pritchard DI, Quinnell RJ, Walsh EA (1995) Immunity in humans to Necator americanus: IgE, parasite weight and fecundity. Parasite Immunol 17:71–75. doi:10.1111/j.1365-3024.1995.tb00968.x
Ramos PW (2013) Investigation of hookworm (Uncinaria sp.) parasites in free-ranging Australian fur seals (Arctocephalus pusillus doriferus). Masters Dissertation, The University of Melbourne
Rogerson JD, Fairbanks WS, Cornicelli L (2008) Ecology of gastropod and bighorn sheep hosts of lungworm on isolated, semiarid mountain ranges in Utah, USA. J Wildl Dis 44:28–44. doi:10.7589/0090-3558-44.1.28
Seguel M, Paredes E, Pavés H, Molina R, Henríquez F, De Groote F, Schlatter R (2011) Pathological findings in South American fur seal pups (Arctocephalus australis gracilis) found dead at Guafo Island, Chile. J Comp Pathol 145:308–317. doi:10.1016/j.jcpa.2011.01.006
Seguel M, Paredes E, Pavés H, Gottdenker NL (2013) Capture-induced stress cardiomyopathy in South American fur seal pups (Arctophoca australis gracilis). Mar Mamm Sci. doi:10.1111/mms.12079
Sepúlveda MS, Alcaíno H (1993) Helminthological fauna of the Juan Fernandez fur seal, Arctocephalus philippii (Peters, 1866). Parasitol Dia 17:19–24
Sepúlveda MS (1998) Hookworms (Uncinaria sp.) in Juan Fernandez fur seal pups (Arctocephalus philippii) from Alejandro Selkirk Island, Chile. J Parasitol 84:1305–1307. doi:10.2307/3284700
Shaw JL, Moss R (1989) The role of parasite fecundity and longevity in the success of Trichostrongylus tenuis in low density red grouse populations. Parasitology 99:253–258. doi:10.1017/S0031182000058704
Shoop WL (1991) Vertical transmission of helminths: hypobiosis and amphiparatenesis. Parasitol Today 7:51–54. doi:10.1016/0169-4758(91)90189-U
Smith K, Acevedo-Whitehouse K, Pedersen A (2009) The role of infectious diseases in biological conservation. Anim Conserv 12:1–12. doi:10.1111/j.1469-1795.2008.00228.x
Spada E, Proverbio D, Della Pepa A, Domenichini G, Bagnagatti De Giorgi G, Traldi G, Ferro E (2013) Prevalence of faecal-borne parasites in colony stray cats in northern Italy. J Feline Med Surg 15:672–677. doi:10.1177/1098612x12473467
Spraker TR, Delong RL, Lyons ET, Melin SR (2007) Hookworm enteritis with bacteremia in California sea lion pups on San Miguel Island. J Wildl Dis 43:179. doi:10.7589/0090-3558-43.2.179
Stirling I (1972) Observations on the Australian sea lion, Neophoca cinerea (Peron). Aust J Zool 20:271–279. doi:10.1071/ZO9720271
Stoye M (1973) Untersuchungen über die möglichkeit pränataler und galaktogener infektionen mit Ancylostoma caninum Ercolani 1859 (Ancylostomidae) beim hund. Zbl Vet Med B 20:1–39. doi:10.1111/j.1439-0450.1973.tb01098.x
Telfer S, Bennett M, Bown K, Cavanagh R, Crespin L, Hazel S, Jones T, Begon M (2002) The effects of cowpox virus on survival in natural rodent populations: increases and decreases. J Anim Ecol 71:558–568. doi:10.1046/j.1365-2656.2002.00623.x
Telfer S, Bennett M, Bown K, Carslake D, Cavanagh R, Hazel S, Jones T, Begon M (2005) Infection with cowpox virus decreases female maturation rates in wild populations of woodland rodents. Oikos 109:317–322. doi:10.1111/j.0030-1299.2005.13734.x
Thompson RCA, Lymbery AJ, Smith A (2010) Parasites, emerging disease and wildlife conservation. Int J Parasitol 40:1163–1170. doi:10.1016/j.ijpara.2010.04.009
Tompkins DM, Dunn AM, Smith MJ, Telfer S (2011) Wildlife diseases: from individuals to ecosystems. J Anim Ecol 80:19–38. doi:10.1111/j.1365-2656.2010.01742.x
Twisleton-Wykeham-Fiennes RN (1966) The Zoological Society of London Report of the Society's Pathologist for the year 1963. J Zool 148:341–362. doi:10.1111/j.1469-7998.1966.tb02955.x
Vicente J, Fernández de Mera IG, Gortazar C (2006) Epidemiology and risk factors analysis of elaphostrongylosis in red deer (Cervus elaphus) from Spain. Parasitol Res 98:77–85. doi:10.1007/s00436-005-0001-2
Walker MJ, Jacobs DE (1982) Epidemiology of Uncinaria stenocephala infections in greyhound breeding kennels. Vet Parasitol 10:317–321. doi:10.1016/0304-4017(82)90083-8
Acknowledgements
We thank the staff at Seal Bay, Department of Environment, Water and Natural Resources (DEWNR), South Australia for logistical support, field assistance and the collection of deceased pups, in particular Clarence Kennedy and Janet Simpson. We thank Rebecca McIntosh of La Trobe University and Phillip Island Nature Parks for the implementation of the comprehensive microchipping program at Seal Bay and Clarence Kennedy of DEWNR and Simon Goldsworthy of the South Australian Research and Development Institute for the ongoing maintenance of this monitoring program. Thank you to Tony Jones and Adam Kemp of Protec Marine, Port Lincoln, South Australia, for providing transport and logistical support for field work at Dangerous Reef. We also thank Evelyn Hall of The University of Sydney and Michael Terkildsen for statistical advice; Denise McDonell of The University of Sydney for laboratory assistance; and volunteers and colleagues for field assistance: Liisa Ahlstrom, Loreena Butcher, Michael Edwards, Simon Goldsworthy, Benjamin Haynes, Claire Higgins, Janet Lackey, Zoe Larum, Theresa Li, Andrew Lowther, Rebecca McIntosh, Paul Rogers, Laura Schmertmann, Adrian Simon, Ryan Tate, Michael Terkildsen, Mark Whelan, Peter White, Sy Woon and Mariko Yata. This work was supported by the Australian Marine Mammal Centre, Department of the Environment, Australian Government (grant number 09/17). All samples were collected under the Government of South Australia Department of Environment, Water and Natural Resources Wildlife Ethics Committee approvals (3–2008 and 3–2011) and Scientific Research Permits (A25008/4-8).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Marcus, A.D., Higgins, D.P. & Gray, R. Epidemiology of hookworm (Uncinaria sanguinis) infection in free-ranging Australian sea lion (Neophoca cinerea) pups. Parasitol Res 113, 3341–3353 (2014). https://doi.org/10.1007/s00436-014-3997-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3997-3