Abstract
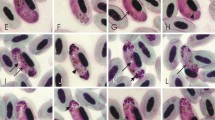
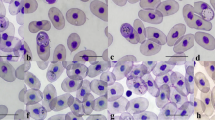
During a surveillance programme on avian influenza in wild birds in the east of Colombia, 42 % of examined wild black-bellied whistling ducks (Dendrocygna autumnalis) were infected with undescribed Haemoproteus sp., which macrogametocytes possess one or several huge (2.5 μm in largest diameter) conspicuous roundish vacuoles, a unique character of avian haemoproteids. This parasite is named Haemoproteus (Parahaemoproteus) macrovacuolatus and described here using data on the morphology of its gametocytes, host cells and sequences of the complete mitochondrial genome and cytochrome b fragments. Illustrations of blood stages of the new species and DNA sequence information are provided. The phylogenetic analysis identified a closely related lineage C033, reported in South Asian ducks belonging to Dendrocygna. We also found that all Haemoproteus lineages from Passeriformes conformed a monophyletic group. Whereas we cannot exclude that this pattern could be an artefact of the limited taxonomic sampling in non-passeriform birds, thus this finding is worthy of attention. This study adds to our knowledge of the phylogenetic relationships among species of avian haemoproteids and describes a new haemoparasite in a non-passerine host.



Similar content being viewed by others
References
Aguirre AA, McLean RG, Cook RS (1992) Experimental inoculation of three arboviruses in black-bellied whistling ducks (Dentrocygna autumnalis). J Wildl Dis 28:521–525
Atkinson CT (2008) Haemoproteus. In: Atkinson CT, Thomas NJ, Hunter BC (eds) Parasitic diseases of wild birds. Wiley-Blackwell, Ames, pp 13–35
Beadell J, Gering E, Austin J, Dumbacher J, Peirce MA (2004) Prevalence and differential host-specificity of two avian blood parasite genera in the Australo-Papuan region. Mol Ecol 13:3829–3844
Beadell J, Fleisher R (2005) A restriction enzyme-based assay for distinguishing between avian haematozoa. J Parasitol 91:683–685
Bennett GF, Garnham PCC, Fallis AM (1965) On the status of the genera Leucocytozoon Ziemann, 1898 and Haemoproteus Kruse, 1890 (Haemosporidia: Leucocytozoidae and Haemoproteidae). Can J Zool 43:927–932. doi:10.1139/z65-096
Bennett GF, Nieman DJ, Turner B, Kuyt E, Whiteway M, Greiner EC (1982) Blood parasites of prairie anatids and their implication in waterfowl management in Alberta and Saskatchewan. J Wildl Dis 18:287–296
Bennett GF, Turner B, Whiteway M (1984) Avian Haemoproteidae. 18. Haemoproteus greineri, a new species of haemoproteid from the waterfowl family Anatidae. Can J Zool 62:2290–2292. doi:10.1139/z84-333
Bennett GF, Peirce MA (1988) Morphological form in the avian Haemoproteidae and an annotated checklist of the genus Haemoproteus Kruse, 1890. J Nat Hist 22:1683–1696. doi:10.1080/00222938800771061
Bennett GF (1993) Haemoproteus gabaldoni n. sp. (Apicomplexa: Haemoproteidae) from the Muscovy duck Cairinamoschata (Aves: Anatidae). Syst Parasitol 25:119–123
Bennett GF, Stotts VD, Bateman MC (1991) Blood parasites of black ducks and other anatids from Labrador and insular Newfoundland. Can J Zool 69:1405–1407. doi:10.1139/z91-198
BirdLife International (2012) Dendrocygna autumnalis. In: IUCN 2013. IUCN Red list of threatened species. Version 2013.1. www.iucnredlist.org. Accessed 11 August 2013
Carrasquilla M, Guhl F, Zipa Y, Ferro C, Pardo R, Cabrera O, Santamaria E (2010) Breeding sites of Culicoides pachymerus Lutz in the Magdalena River basin, Colombia. Mem Inst Oswaldo Cruz 105:216–219
Cumming G, Shepard E, Okanga S, Caron A, Ndlovu M, Peters J (2013) Host associations, biogeography, and phylogenetics of avian malaria in southern African waterfowl. Parasitology 140:193–201. doi:10.1017/S0031182012001461
Desser SS (1967) Schizogony and gametogony of Leucocytozoon simondi and associated reactions in the avian host. J Protozool 14:244–254
Desser SS, Fallis AM, Garnham PC (1968) Relapses in ducks chronically infected with Leucocytozoonsimondi and Parahaemoproteusnettionis. Can J Zool 46:281–285. doi:10.1139/z68-041
Ellis TM, Leung CY, Chow MK, Bissett LA, Wong W et al (2004) Vaccination of chickens against H5N1 avian influenza in the face of an outbreak interrupts virus transmission. Avian Pathol 33:405–412
Fallis AM, Wood DM (1957) Biting midges (Diptera: Ceratopogonidae) as intermediate hosts for Haemoproteus of ducks. Can J Zool 35:425–435. doi:10.1139/z57-033
Fallis AM, Bisset SA, Allison FR (1976) Leucocytozoon tawaki n. sp. (Eucoccida: Leucocytozoidae) from the penguin Eudyptespachyrhynchus, and preliminary observations on its development in Austrosimulium spp. (Diptera: Simuliidae). N Z J Zool 3:11–16
Gabaldon A, Ulloa G, Gomez de Montcourt A (1975) Encuestasobre malaria aviaria en Venezuela. Resultados del segundo año. Bol Dir Malariol San Amb XV(3):73–92
Gabaldon A, Ulloa G (1976) Encuesta sobre malaria aviaria en Venezuela: resultados del tercer y último año. Bol Dir Malariol San Amb XVI(2):107–117
Greiner EC, Bennett GF, White EM, Coombs RF (1975) Distribution of the avian hematozoa of North America. Can J Zool 53:1762–1787
Hamer GL, Anderson TK, Berry GE, Makohon-Moore AP, Crafton JC, Brawn JD, Dolinski AC, Krebs BL, Ruiz MO, Muzzall PM, Goldberg TL, Walker ED (2013) Prevalence of filarioid nematodes and trypanosomes in American robins and house sparrows, Chicago USA. Int J Parasitol Parasites Wildl 2:42–49. doi:10.1016/j.ijppaw.2012.11.005
Heard DJ, MulcahyDM ISA, Rizzolo DJ, Greiner EC, Hall J, Ip H, Esler D (2008) A blood survey of elements, viral antibodies, and hemoparasites in Wintering Harlequin Ducks (Histrionicus histrionicus) and Barrow’s Goldeneyes (Bucephalaislandica). J Wildl Dis 44:486–493
Hellgren O, Waldenström J, Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol 90:797–802
Hénaux V, Parmley J, Soos C, Samuel MD (2013) Estimating transmission of avian influenza in wild birds from incomplete epizootic data: implications for surveillance and disease spread. J Appl Ecol 50:223–231. doi:10.1111/1365-2664.12031
Hilty SL, Brown WL (1986) A guide to the birds of Colombia. Princeton University Press, New Jersey
Julian RJ, Galt DE (1980) Mortality in Muscovy ducks (Cairinamoschata) caused by Haemoproteus infection. J Wildl Dis 16:39–44
Karadjian G, Puech M-P, Duval L, Chavatte J-M, Snounou G, Landau I (2013) Haemoproteus syrnii in Strix aluco from France: morphology, stages of sporogony in a hippoboscid fly, molecular characterization and discussion on the identification of Haemoproteus species. Parasite 20:32. doi:10.1051/parasite/2013031
Khan RA, Fallis AM (1968) Comparison of infections with Leucocytozoonsimondi in black ducks (Anasrubripes), mallards (Anasplatyrhynchos), and white Pekins (Anasbochas). Can J Zool 46:773–780
Komar N, Clark GG (2006) West Nile Virus activity in Latin America and the Caribbean. Rev Panam Salud Publica 19:112–117
Križanauskienė A, Hellgren O, Kosarev V, Sokolov L, Bensch S, Valkiūnas G (2006) Variation in host specificity between species of avian hemosporidian parasites: evidence from parasite morphology and cytochrome b gene sequences. J Parasitol 92:1319–1324
Kučera J, MarjánkováK RV, Vítovec J (1982) Haemosporidiosis as a fatal disease in Muscovy ducks (Cairina moschata) in South Bohemia. Folia Parasitol 29:193–200
Martínez-De-La-Puente J, Merino S, Tomás G, Moreno J, Morales J, Lobato E, García- Fraile S, Belda EJ (2010) The blood parasite Haemoproteus reduces survival in a wild bird: a medication experiment. Biol Lett 6:663–665. doi:10.1098/rsbl.2010.0046
Martinsen S, Perkins S, Schall J (2008) A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol 47:261–273. doi:10.1016/j.ympev.2007.11.012
Marzal A, Bensch S, Reviriego M, Balbontin J, de Lope F (2008) Effects of malaria double infection in birds: one plus one is not two. J Evol Biol 21:979–987. doi:10.1111/j.1420-9101.2008.01545.x
Muñoz E, Ferrer D, Molina R, Adlard RD (1999) Prevalence of haematozoa in birds of prey in Catalonia, northeast Spain. Vet Rec 144:632–636
Newman CM, Cerutti F, Anderson TK, Hamer GL, Walker ED, Kitron UD, Ruiz MO, Brawn JD, Goldberg TL (2011) Culex flavivirus and West Nile virus mosquito co-infection and positive association in Chicago USA. Vector Borne Zoonotic Dis 11:1099–1105. doi:10.1089/vbz.2010.0144
Palinauskas V, Valkiūnas G, Bensch S, Bolshakov VC (2011) Plasmodium relictum (lineage SGS1) and Plasmodium ashfordi (lineage GRW2). The effects of the coinfection on experimentally infected passerine birds. Exp Parasitol 127:527–533. doi:10.1016/j.exppara.2010.10.007
Pacheco MA, Battistuzzi FU, Junge RE, Cornejo OE, Williams CV, Landau I, Rabetafika L, Snounou G, Jones-Engel L, Escalante AA (2011) Timing the origin of human malarias: the lemur puzzle. BMC Evol Biol 11:299. doi:10.1186/1471-2148-11-299
Pennycott TW, Park A, Cinderey RN, Mather HA, Foster G (2002) Salmonella enterica subspecies enterica serotype Typhimurium and Escherichia coli O86 in wild birds at two garden sites in southwest Scotland. Vet Rec 151:563–567
Pereda AJ, Uhart M, Perez AA, Zaccagnini ME, La Sala L, Decarre J, Goijmand A, Solari L, Suarez R, Craig MI, Vagnozzi A, Rimondi A, König G, Terrera MV, Kaloghlianc A, Song H, Sorrell EM, Perez DR (2008) Avian influenza virus isolated in wild waterfowl in Argentina: evidence of a potentially unique phylogenetic lineage in South America. Virology 378:363–370. doi:10.1016/j.virol.2008.06.010
Pung OJ, Maxwell NE, Greiner EC, Robinette JR, Thul JE (1997) Haemoproteus greineri in wood ducks from the Atlantic Flyway. J Wildl Dis 33:355–358
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg180
Sambrook J, Fritsch EF, Maniatis T (1989) Chapter 6 Preparation and analysis of eukaryotic genomic DNA. In: Molecular cloning a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, New York, pp 6.4–6.12
Skírnisson K, Kolářová L (2008) Diversity of bird schistosomes in anseriform birds in Iceland based on egg measurements and egg morphology. Parasitol Res 103:43–50. doi:10.1007/s00436-008-0925-4
Scorza JV (1971) Electron microscope study of the blood stages of Plasmodium tropiduri, a lizard malaria parasite. Parasitology 63:1–20
Sibley LD, Werner JK (1984) Susceptibility of Pekin and Muscovy ducks to Haemoproteus nettionis. J Wildl Dis 20:108–113
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi:10.1093/molbev/msr121
Tella JL, Blanco G, Forero MG, Gajon A, Donázar JA et al (1999) Habitat, world geographic range, and embryonic development hosts explain the prevalence of avian hematozoa at small spatial and phylogenetic scales. Proc Natl Acad Sci 96:1785–1789
Telford SR (2009) Hemoparasites of the Reptilia: color atlas and text. CRC Press, Boca Raton
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton
Valkiūnas G, Iezhova TA, Loiseau C, Smith TB, Sehgal RNM (2008) New malaria parasites of the subgenus Novyella in African rainforest birds, with remarks on their high prevalence, classification and diagnostics. Parasitol Res 104:1061–1077. doi:10.1007/s00436-008-1118-x
Williams NA, Bennett GF (1980) Avian haemoproteidae. 13. The haemoproteids of the ducks and geese (Anatidae). Can J Zool 58:88–93. doi:10.1139/z80-012
White EM, Greiner EC, Bennett GF, Herman CM (1978) Distribution of hematozoa of Neotropical birds. Rev Biol Trop 26:43–102
Acknowledgments
We thank J. Perez, I. Bernal and F. González who conducted field work, C. Saavedra for project coordination, P. Franco, Head of Colombia Program of Environmental and Sustainable Development Ministry, C. Rodríguez, Professional of Forests, Biodiversity and Ecosystem Services Office, T. Tovar, P. Quiroga, A. González and I. Lotta for their help with laboratory analyses, T. Iezhova for technical assistance during preparation of Figs. 1–24. Funding for field work was provided by the Wildlife Conservation Society. A. A. Escalante is supported by the grant R01GM080586 from the US National Institute of Health. This study was conducted under the Colombian surveillance programme (agreement no. 27 of 2012) for avian influenza and other avian diseases established by the Ministry of Environment and Sustainable Development (MADS) from the Republic of Colombia and the Wildlife Conservation Society (WCS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matta, N.E., Pacheco, M.A., Escalante, A.A. et al. Description and molecular characterization of Haemoproteus macrovacuolatus n. sp. (Haemosporida, Haemoproteidae), a morphologically unique blood parasite of black-bellied whistling duck (Dendrocygna autumnalis) from South America. Parasitol Res 113, 2991–3000 (2014). https://doi.org/10.1007/s00436-014-3961-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3961-2