Abstract
Background
Lipid quantity and quality have been shown to affect serum cholesterol, adipose and serum leptin levels during prenatal and postnatal dietary supplementation of adult rats. Maternal protein deficiency during pregnancy and lactation also affects polyunsaturated fatty acid (PUFA) levels in the offspring. The aim of the present study was to analyze the effect of α-linolenic acid (ALA; n-3) on n-3 PUFA accretion, lipid profile, leptin levels and adipose growth in normal and protein-restricted (deficient) dams and their suckling pups.
Methods
Garden cress oil rich in ALA (32 %) was supplemented in the normal and protein-restricted (10 %) diets and fed to rats for 8 weeks prior to gestation and during lactation. PUFA, cholesterol, triglycerides, leptin levels and retroperitoneal white adipose tissue weight (WAT) of the dams and the pups were analyzed at 3 weeks after delivery.
Results
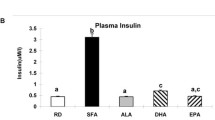
The serum cholesterol levels were remarkably decreased (p < 0.01), and the n-3 PUFA levels were markedly increased (p < 0.05) in the pups of lactating normal and protein-deficient dams supplemented with ALA. Triglycerides were unaltered in the dams and the pups of different dietary groups. Serum leptin levels and relative WAT weights were lower (p < 0.01) in the pups of the ALA-supplemented normal and protein-deficient dams.
Conclusion
Maternal supplementation of ALA in normal and protein-restricted diets modulates n-3 PUFA levels, cholesterol, leptin levels and also adipose growth in the suckling offspring.


Similar content being viewed by others
References
Connor WE (2000) Importance of n-3 fatty acids in health and disease. Am J Clin Nutr 71:171S–175S
Simopoulos AP (1991) Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr 54:438–463
Spector AA (1999) Essentiality of fatty acids. Lipids 34:S1–S3
Burdge C, Wooton SA (2002) Conversion of α-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr 88:411–421
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56:365–379
Ruxton CH, Reed SC, Simpson MJ (2004) The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet 17:449–459
Sprecher H, Luthria DL, Mohammed BS, Baykousheva SP (1995) Reevaluation of the pathways for the biosynthesis of polyunsaturated fatty acids. J Lipid Res 36:2471–2477
Rodriguez A, Sarda P, Nessmann C, Boulot P, Leger CL, Descomps B (1998) Delta 6- and delta5-desaturase activities in the human fetal liver: kinetic aspects. J Lipid Res 39:1825–1832
Jensen RG (1999) Lipids in human milk. Lipids 34:1243–1271
Korotkova M, Gabrielsson B, Holmang A, Larsson BM, Hanson LA, Strandvik B (2005) Gender related long-term effects in adult rats by perinatal dietary ratio of n-6/n-3 fatty acids. Am J Physiol Regul Integr Comp Physiol l288:R575–R579
Moon RJ, Harvey NC, Robinson SM, Ntani G, Davies JH et al (2013) Maternal plasma polyunsaturated fatty acid status in late pregnancy is associated with offspring body composition in childhood. J Clin Endocrinol Metab 98:299–307
Ailhaud G, Guesnet P (2004) Fatty acid composition of fats is an early determinant of childhood obesity: a short review and an opinion. Obes Rev 5:21–26
Donahue SMA, Rifas-Shiman SL, Gold DR, Jouni ZE, Gillman MW, Oken E (2011) Prenatal fatty acid status and child adiposity at age 3 year: results from a US pregnancy cohort. Am J Clin Nutr 93:780–788
Kopecky J, Rossmeis M, Flachs P, Kuda O et al (2009) N-3 PUFA: bioavailability and modulation of adipose tissue function. Proc Nutr Soc 68:361–369
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432
Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM (1995) Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight reduced subjects. Nat Med 1:1155–1161
Considine RV, Sinha MK, Heiman ML, Kriaugiunas A, Stephens TW, Nyge MR, Ohannesian JP, Margo CC, McKee LJ, Bauer TL, Caro JF (1996) Serum immune-reactive leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295
Raclot T, Groscolas R, Langin D, Ferre P (1997) Site-specific regulation of gene expression by n-3 polyunsaturated fatty acids in rat white adipose tissues. J Lipid Res 38:1963–1972
Nadkarni KM (1954) The Indian materia medica, 3rd edn. Dhootapa Peshwar Prakashan Ltd., Panvel
Anonymous (1972) The wealth of Indian raw materials, vol 9. Publication and Information Directorate, CSIR, New Delhi, pp 71–72
Diwakar BT, Dutta PK, Lokesh BR, Naidu KA (2010) Physicochemical properties of garden cress (Lepidium sativum L.) seed oil. J Am Oil Chem Soc 87:539–548
Diwakar BT, Dutta PK, Lokesh BR, Naidu KA (2008) Bio-availability and metabolism of n-3 fatty acid rich garden cress (Lepidium sativum) seed oil in albino rats. Prostaglandins Leukot Essent Fatty Acids 78:123–130
Diwakar BT, Lokesh BR, Naidu KA (2011) Modulatory effect of α-linolenic acid-rich garden cress (Lepidium sativum L) seed oil on inflammatory mediators in adult albino rats. Br J Nutr 106:530–539
Reddy KVK, Maheswaraiah A, Naidu KA (2014) Rice bran oil and garden cress (Lepidium sativum L) seed oil attenuate murine model of colitis. Int J Colorectal Dis 29:267–269
Thefeld W, Hoffmeister H, Busch EW, Koller PU, Vollmar J (1974) Dtsch Med Wochenschr 99:343–351
Tietz NW, Burtis CA, Duncan P, Ervin K, Petitclerc CJ, Rinker AD et al (1983) A reference method for measurement of alkaline phosphatase activity in human serum. Clin Chem 29:751–761
Talke H, Schubert GE (1965) Klin Wochenschr 43:174
Jacobs NJ, Vandemark PJ (1960) The purification and properties of the alpha-glycerophosphate-oxidizing enzyme of Streptococcus faecalis 10C1. Arch Biochem Biophys 88:250–255
Allain CC, Lucy SP et al (1974) Enzymatic determination of total cholesterol. Clin Chim 20:470
Rifai N, Warnick GR (1994) Laboratory measurement of lipids, lipoproteins and apolipoproteins. AACC Press, Washington
Searcy RL, Bergquist LM (1960) A new color reaction for the quantification of serum cholesterol. Clin Chim Acta 5:192–199
Fletcher MJ (1968) A colorimetric method for estimating serum triglycerides. Clin Chim Acta 22:303–307
Folch J, Lees M, Sloane-Stanley GH (1956) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Morrison WR, Smith LM (1964) Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron trifluoride methanol. J Lipid Res 5:600–608
Cottin SC, Sanders TA, Hall WL (2011) The differential effects of EPA and DHA on cardiovascular risk factors. Proc Nutr Soc 70:215–231
Grimsgaard S, Bona KH, Bjarne JH, Nordoy A (1997) Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol effects but divergent effects on serum fatty acids. Am J Clin Nutr 66:649–659
Troina AA, Figueiredo MS, Moura EG, Boaventura GT et al (2010) Maternal flaxseed diet during lactation alters milk composition and programs the offspring body composition, lipid profile and sexual function. Food Chem Toxicol 48:697–703
Korotkova M, Gabrielsson B, Lonn M, Hanson LA, Strandvik B (2002) Leptin levels in rat offspring are modified by the ratio of linoleic to alpha-linolenic acid in the maternal diet. J Lipid Res 43:1743–1749
Wiesenfeld PW, Babu US, Collins TFX, Sprando R et al (2003) Flaxseed increased a-linolenic and eicosapentaenoic acid and decreased arachidonic acid in serum and tissues of rat dams and offspring. Food Chem Toxicol 41:841–855
Ozias Marlies K, Susan E, Carlson A, Levant B (2007) Maternal parity and diet (n-3) polyunsaturated fatty acid concentration influence accretion of brain phospholipid docosahexaenoic acid in developing rats. J Nutr 137:125–129
Zambrano E, Bautista CJ, Deas M, Martınez-Samayoa PM, Gonzalez-Zamorano M, Ledesma H, Morales J, Larrea F, Nathanielsz PW (2006) A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol 571:221–230
Passes MCF, Ramos CF, Moura EG (2000) Short and long term effects of malnutrition in rats during lactation on the body weight of offspring. Nutr Res 20(11):1603–1612
Burdge GC, Delange E, Dubois L, Dunn RL, Mark A, Alan H, Jackson A, Calder PC (2003) Effect of reduced maternal protein intake in pregnancy in the rat on the fatty acid composition of brain, liver, plasma, heart and lung phospholipids of the offspring after weaning. Br J Nutr 90:345–352
Torres N, Bautista CJ, Tovar AR, Ordaz G et al (2010) Protein restriction during pregnancy affects maternal liver lipid metabolism and fetal brain lipid composition in the rat. Am J Physiol Endocrinol Metab 298:E270–E277
Burdge GC, Dunn RL, Wootton SA, Jackson AA (2002) Effect of reduced dietary protein intake on hepatic and plasma essential fatty acid concentrations in the adult female rat: effect of pregnancy and consequences for accumulation of arachidonic and docosahexaenoic acids in fetal liver and brain. Br J Nutr 88:379–387
Ahima RS, Flier JS (2000) Leptin. Annu Rev Physiol 62:413–437
Acknowledgments
The authors thank Director, Prof. Ram Rajsekharan, CSIR-CFTRI, Mysore, for his support and encouragement in the present study. Mr. K.V.K Reddy, CSIR senior research fellow, gratefully acknowledges the financial assistance from Council of Scientific and Industrial Research (CSIR), New Delhi, in carrying out these investigations. KAN gratefully acknowledges the financial support in the form of a Project (SR/SO/HS-0005/2010) awarded by Department of Science and Technology (DST), New Delhi, India.
Conflict of interest
Authors do not have any financial conflict of interest. We have no commercial arrangement with companies that market garden cress oil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, K.V.K., Naidu, K.A. Maternal supplementation of α-linolenic acid in normal and protein-restricted diets modulate lipid metabolism, adipose tissue growth and leptin levels in the suckling offspring. Eur J Nutr 54, 761–770 (2015). https://doi.org/10.1007/s00394-014-0755-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0755-3