Abstract
Purpose
High-grade gliomas are closely related to the mesenchymal phenotype which might be explained by unorthodox differentiation of glioma cancer stem cells (gCSCs). We reasoned that other non-neural stem cells, especially mesenchymal stem cells (MSCs), might play a role in expresssing mesenchymal phenotype of high-grade gliomas. Thus we hypothesized that cells resembling MSCs exist in glioma specimens.
Methods
We created a mouse (m) orthotopic glioma model using human gCSCs. Single-cell suspensions were isolated from glioma specimens and cultured according to the methods for mMSCs or gliomaspheres. These cells were analyzed by fluorescence-activated cell sorting (FACS) for surface markers associated with mMSCs or gCSCs. Glioma stroma (GS)-MSCs were exposed to mesenchymal differentiation conditions. To decide the location of GS-MSCs, sections of orthotopic glioma models were analyzed by immunofluorescent labeling.
Results
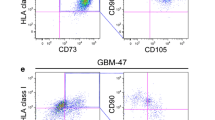
GS-MSCs were isolated which were morphologically similar to mMSCs. FACS analysis showed that the GS-MSCs had similar surface markers to mMSCs (stem cell antigen-1 [Sca-1]+, CD9+, CD45−, CD11b−, CD31−, and nerve/glial antigen 2 [NG2]−). GS-MSCs were capable of mesenchymal differentiation. Immunofluorescent labeling indicated that GS-MSCs are located around blood vessels, are distinct from endothelial cells, and have features that partially overlap with vascular pericytes.
Conclusions
Our results indicate that cells similar to mMSCs exist in glioma specimens. The GS-MSCs might be located around vessels, which suggests that GS-MSCs may provide the mesenchymal elements of the vascular niche. GS-MSCs may represent non-neural stem cells that act as an important source of mesenchymal elements, particularly during the growth of gliomas.







Similar content being viewed by others
References
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63:5821–5828
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Hoelzinger DB, Demuth T, Berens ME (2007) Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst 99:1583–1593
Oliver L, Olivier C, Marhuenda FB, Campone M, Vallette FM (2009) Hypoxia and the malignant glioma microenvironment: regulation and implications for therapy. Curr Mol Pharmacol 2:263–284
Kanamori M, Kawaguchi T, Berger MS, Pieper RO (2006) Intracranial microenvironment reveals independent opposing functions of host alphaVbeta3 expression on glioma growth and angiogenesis. J Biol Chem 281:37256–37264
Stewart PA, Farrell CL, Del Maestro RF (1991) The effect of cellular microenvironment on vessels in the brain. Part 1: vessel structure in tumour, peritumour and brain from humans with malignant glioma. Int J Radiat Biol 60:125–130
Kievit FM, Florczyk SJ, Leung MC, Veiseh O, Park JO, Disis ML, Zhang M (2010) Chitosan-alginate 3D scaffolds as a mimic of the glioma tumor microenvironment. Biomaterials 31:5903–5910
Galderisi U, Cipollaro M, Giordano A (2006) Stem cells and brain cancer. Cell Death Differ 13:5–11
Fomchenko EI, Holland EC (2005) Stem cells and brain cancer. Exp Cell Res 306:323–329
Xouri G, Christian S (2010) Origin and function of tumor stroma fibroblasts. Semin Cell Dev Biol 21:40–46
Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 21:2683–2710
Holland EC (2000) Glioblastoma multiforme: the terminator. Proc Natl Acad Sci USA 97:6242–6244
Freije WA, Castro-Vargas FE, Fang Z, Horvath S, Cloughesy T, Liau LM, Mischel PS, Nelson SF (2004) Gene expression profiling of gliomas strongly predicts survival. Cancer Res 64:6503–6510
Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K (2006) Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 9:157–173
Tso CL, Shintaku P, Chen J, Liu Q, Liu J, Chen Z, Yoshimoto K, Mischel PS, Cloughesy TF, Liau LM, Nelson SF (2006) Primary glioblastomas express mesenchymal stem-like properties. Mol Cancer Res 4:607–619
Rieske P, Golanska E, Zakrzewska M, Piaskowski S, Hulas-Bigoszewska K, Wolanczyk M, Szybka M, Witusik-Perkowska M, Jaskolski DJ, Zakrzewski K, Biernat W, Krynska B, Liberski PP (2009) Arrested neural and advanced mesenchymal differentiation of glioblastoma cells—comparative study with neural progenitors. BMC Cancer 9:54
Ricci-Vitiani L, Pallini R, Larocca LM, Lombardi DG, Signore M, Pierconti F, Petrucci G, Montano N, Maira G, De Maria R (2008) Mesenchymal differentiation of glioblastoma stem cells. Cell Death Differ 15:1491–1498
Boerman RH, Anderl K, Herath J, Borell T, Johnson N, Schaeffer-Klein J, Kirchhof A, Raap AK, Scheithauer BW, Jenkins RB (1996) The glial and mesenchymal elements of gliosarcomas share similar genetic alterations. J Neuropathol Exp Neurol 55:973–981
Hall B, Dembinski J, Sasser AK, Studeny M, Andreeff M, Marini F (2007) Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol 86:8–16
Roorda BD, ter Elst A, Kamps WA, de Bont ES (2009) Bone marrow-derived cells and tumor growth: contribution of bone marrow-derived cells to tumor micro-environments with special focus on mesenchymal stem cells. Crit Rev Oncol Hematol 69:187–198
Stagg J (2008) Mesenchymal stem cells in cancer. Stem Cell Rev 4:119–124
Lal S, Lacroix M, Tofilon P, Fuller GN, Sawaya R, Lang FF (2000) An implantable guide–screw system for brain tumor studies in small animals. J Neurosurg 92:326–333
Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, Chen J, Hentschel S, Vecil G, Dembinski J, Andreeff M, Lang FF (2005) Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res 65:3307–3318
Tropel P, Noel D, Platet N, Legrand P, Benabid AL, Berger F (2004) Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res 295:395–406
Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED (2006) Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells 24:1054–1064
Kang SG, Shinojima N, Hossain A, Gumin J, Yong RL, Colman H, Marini F, Andreeff M, Lang FF (2010) Isolation and perivascular localization of mesenchymal stem cells from mouse brain. Neurosurgery 67:711–720
Fidler IJ, Poste G (2008) The “seed and soil” hypothesis revisited. Lancet Oncol 9:808
Paget S (1889) The distribution of secondary growths in cancer of the breast. Lancet 133:571–573
Mendoza M, Khanna C (2009) Revisiting the seed and soil in cancer metastasis. Int J Biochem Cell Biol 41:1452–1462
Lin HJ, Zuo T, Chao JR, Peng Z, Asamoto LK, Yamashita SS, Huang TH (2009) Seed in soil, with an epigenetic view. Biochim Biophys Acta 1790:920–924
Marx J (2008) Cancer biology. All in the stroma: cancer’s Cosa Nostra. Science 320:38–41
Liotta LA, Kohn EC (2001) The microenvironment of the tumour–host interface. Nature 411:375–379
Lottaz C, Beier D, Meyer K, Kumar P, Hermann A, Schwarz J, Junker M, Oefner PJ, Bogdahn U, Wischhusen J, Spang R, Storch A, Beier CP (2010) Transcriptional profiles of CD133+ and CD133− glioblastoma-derived cancer stem cell lines suggest different cells of origin. Cancer Res 70:2030–2040
Hall B, Andreeff M, Marini F (2007) The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb Exp Pharmacol 180:263–283
Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D (2008) Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res 68:4331–4339
Dvorak HF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315:1650–1659
Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA (2005) Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation 112:214–223
Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H (2004) BDNF gene-modified mesenchymal stem cells promote functional recovery and reduce infarct size in the rat middle cerebral artery occlusion model. Mol Ther 9:189–197
De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L (2005) Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8:211–226
Bababeygy SR, Cheshier SH, Hou LC, Higgins DM, Weissman IL, Tse VC (2008) Hematopoietic stem cell-derived pericytic cells in brain tumor angio-architecture. Stem Cells Dev 17:11–18
da Silva ML, Caplan AI, Nardi NB (2008) In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26:2287–2299
da Silva ML, Chagastelles PC, Nardi NB (2006) Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 119:2204–2213
Nardi NB (2005) All the adult stem cells, where do they all come from? An external source for organ-specific stem cell pools. Med Hypotheses 64:811–817
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0071299 and 2010-0004506) and a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family affairs, Republic of Korea (1020340).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SM., Kang, SG., Park, NR. et al. Presence of glioma stroma mesenchymal stem cells in a murine orthotopic glioma model. Childs Nerv Syst 27, 911–922 (2011). https://doi.org/10.1007/s00381-011-1396-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-011-1396-y