Abstract
In the resource-limited Arctic environment, vegetation developing near seabird colonies is exceptionally luxuriant. Nevertheless, there are very few detailed quantitative studies of any specific plant species responses to ornithogenic manuring. Therefore, we studied variability of polar scurvygrass Cochlearia groe nlandica individual biomass and leaf width along a seabird influenced gradient determining environmental conditions for vegetation in south-west Spitsbergen. We found seabird colony effect being a paramount factor responsible for augmented growth of C. groenlandica. The species predominated close to the colony and reached the highest mean values of individual biomass (1.4 g) and leaf width (26.6 mm) 10 m below the colony. Its abundance and size declined towards the coast. Both C. groenlandica individual traits significantly decreased with distance from the colony, soil water and organic matter content and increased with guano deposition, soil δ 15N, conductivity, acidity and nitrate, phosphate and potassium ion content. Our study supports the hypothesis that seabirds have fundamental importance for vegetation growth in poor Arctic environment. Highly plastic species such as C. groenlandica may be a useful instrument in detecting habitat condition changes, for instance resulting from climate change.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental conditions in Arctic terrestrial ecosystems are severe, even during summer, through factors including low nutrient and water availability, short growing season, unstable weather conditions and permafrost. Vegetation consists mainly of lichens, mosses and vascular plants of low stature and low productivity (Callaghan 2001; Thomas et al. 2008). At the same time, the adjacent marine environment is highly productive due to vertical mixing of water masses and the high levels of activity of photosynthetic diatoms (Cocks et al. 1998; Stempniewicz 2005; Stempniewicz et al. 2007; Thomas et al. 2008). Terrestrial ecosystems may benefit from this rich neighbour but only in the presence of a vector that enables nutrients transport from the sea.
Seabirds constitute the most important link between these two environments. They feed at sea and breed on land, often in very large colonies. During the summer reproductive season, seabirds deposit considerable amounts of guano, as well as dead birds, chicks, feathers, egg shells and food remnants such as fish and/or crustaceans (Polis et al. 1997; Bokhorst et al. 2007). Stempniewicz (1990, 1992) reports that during one breeding season in Hornsund (south-west Spitsbergen), little auks (Alle alle) deliver ~60 t of guano dry mass per km2 of colony area and ~25 t around the colony. Odasz (1994) gives data of 150 kg guano deposited per day below the cliff inhabited by ca. 6,000 pairs of kittiwakes Rissa tridactyla and Brunnich’s guillemots Uria lomvia in the Krossfjorden region (north-west Spitsbergen). Thanks to decomposers (soil invertebrates and microorganisms) nutrients of marine origin such as nitrogen, phosphorus, potassium and calcium are released into the soil and then assimilated by primary producers (Ryan and Watkins 1989; Cragg and Bardgett 2001; Hodkinson et al. 2002; Smith and Froneman 2008). The amount of biogenic salts delivered by colonial polar seabirds is much larger than that originating from any other source, such as precipitation, sea spray or nitrogen bound by lichens and cyanobacteria (Cocks et al. 1998; Erskine et al. 1998; Bokhorst et al. 2007). Vegetation developing near seabird colonies, called ‘ornithogenic tundra’ in Arctic regions (Eurola and Hakala 1977), forms continuous mats and is exceptionally lush and diverse (Elvebakk 1994; Rønning 1996).
Many studies have demonstrated that seabirds are responsible for augmented vegetation growth through their nutrient input (e.g. Wainright et al. 1998; Wait et al. 2005; Zmudczyńska et al. 2009; Mulder et al. 2011; Smith et al. 2011; Zwolicki et al. 2013). Nonetheless, current knowledge and understanding of seabird impact on tundra productivity remains incomplete. It is widely believed that seabird impact is strongest close to large colonies, decreasing with distance, resulting in changes in soil nutrient availability, water content and the level of seabird physical disturbance. At the community level, plants may respond to these changes through variation in cover, species richness, or diversity, depending on species adaptations, requirements and tolerance (Odasz 1994; Theodose and Bowman 1997; Vidal et al. 2003; Pennings et al. 2005; Wait et al. 2005; Zelenskaya and Khoreva 2006; Smykla et al. 2007). At the individual level, conspecific plants may differ in size, morphology, above- and below-ground biomass, content of nutrients such as nitrogen or phosphorus and thus physiological properties including photosynthetic abilities and productivity (Wainright et al. 1998;Anderson and Polis 1999; Zelenskaya and Khoreva 2006; Madan et al. 2007; Zmudczyńska et al. 2008). Trait information within individual species can directly link the response of a species to variable habitat conditions along environmental gradient and is relatively easy to quantify. Individual variability can be influenced by several environmental factors such as nutrient availability (Wookey et al. 1994; Fonseca et al. 2000; Rubio et al. 2003; Trubat et al. 2006; Morgner and Pettersen 2007; Żółkoś and Meissner 2008, 2010; Khoreva and Mochalova 2009), water content (Meškauskaitė and Naujalis 2006; Trubat et al. 2006), altitude (Taguchi and Wada 2001), light (Bragg and Westoby 2002), wind (Niklas 1996) or grazing (Semmartin and Ghersa 2006). However, there are very few detailed quantitative studies of any specific plant species responses to gradients of seabird influence (but see Zmudczyńska et al. 2008; Żółkoś et al. 2008, 2010). Our first research on this subject concerned the variability of alpine saxifrage Saxifraga nivalis L. along the gradient of soil and tundra variability induced by a seabird colony (Zmudczyńska et al. 2008).
This study focuses on polar scurvygrass, Cochlearia groenlandica L. The species has a circumpolar distribution (Elven et al. 2011) and is widespread and very common throughout Svalbard (Rønning 1996). Nevertheless, its size and morphology differ considerably depending on the location and environmental conditions, which suggests its high plasticity and relatively quick reaction to habitat change (Odasz 1994; Morgner and Pettersen 2007). The aim of the study was to document variability of C. groenlandica individual biomass and leaf width along a seabird influenced gradient determining environmental conditions for vegetation. Positive phenotypic response of the species for seabird induced habitat alteration would support the hypothesis that seabirds have fundamental importance for vegetation growth both on individual and community level in the resource-limited Arctic environment. Cochlearia groenlandica had wider range of occurrence on the studied area than previously investigated S. nivalis; thus, it gave much better opportunity for correlating its biometric features with the seabird impact.
Materials and methods
Study area
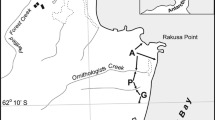
The study was conducted on the north coast of Hornsund (south-west Spitsbergen, Svalbard) in an area of tundra between a large seabird colony and the coast. The colony was situated on Gnålberget cliff (77°01′N 15°52′E) and consisted of Brunnich’s guillemots (Uria lomvia) and black-legged kittiwakes (Rissa tridactyla), both piscivorous species. The area included ca. 500 m between the nesting cliff and seashore, within which a study transect was established, initially with an inclination of 40°–50° directly under the cliff itself and terminating with the flat plain at the coast (Fig. 1). Since the study area was situated along one slope, not higher than ca. 200 m asl, its exposure, insolation and accessibility for herbivores were similar across the whole transect.
Plant communities occurring in the study area are characteristic of well-fertilised bird cliff vegetation (Rønning 1996), with vegetation cover typically 80–98 % across the transect, excepting the seashore itself and sites directly under the cliff that were damaged mechanically by falling rocks. Well-developed vascular plants and algal mats predominated over mosses. The zonal character of vegetation along the colony-sea axis was clearly defined. The plant community developing closest to the cliff and down the slope to ca. 40 m below the colony predominantly comprised C. groenlandica and Prasiola crispa. This gradually changed to communities dominated by Cerastium arcticum-Poa alpina (40–100 m from the cliff), Saxifraga caespitosa (100–160 m) and Festuca rubra (160–240 m). The flatter transect sector adjacent to the shore (240–450 m) was characterised by a Saxifraga oppositifolia–Sanionia uncinata community (Wojtuń, in Zmudczyńska et al. 2009).
In a separate study at this location, we defined a transect situated on a topographically similar area but beyond the routine flight route of the seabirds and hence under minimal seabird influence (Zmudczyńska et al. 2012; Zwolicki et al. 2013). This was also on Gnålberget slope ca. 300 m away from the colony and with the same inclination and sampling plots design. Across this transect, there were no gradual changes in soil physical and chemical properties (Zwolicki et al. 2013) nor in plant communities (Zmudczyńska et al. 2012) along the slope. Vegetation was limited with lower total cover and a higher proportion of mosses, lichens and cyanobacteria. The slope was almost entirely covered with a Sanionia uncinata–Tortula ruralis community, with the exception of the seashore part of the transect that hosted a Sanionia uncinata–Straminergon stramineum community. However, C. groenlandica contributed much less than 1 % of the total cover in sampling plots 1–5, 7 and 8 and was completely absent within sampling plots 6, 9 and 10 of this transect. The sparse individuals were of similar size to that of plants growing in the coastal sector of the current study area, whenever they occurred along the transect.
Study species
Cochlearia groenlandica (Family: Brassicaceae) is a widespread circumpolar, mainly high-Arctic forb. It may occur in habitats including wet meadows, hummocks, around the margin of ponds, depressions of low-centred polygons, along streams, river terraces, ridges and seashores. In these habitats, it is found growing on substrata including rocks, gravel, till, moss, soils with low organic content and acidic, nitrophilous or non-calcareous characteristics (Rønning 1996; Aiken et al. 2007; Elven et al. 2011). Individual plants live 2–5 years. Individual leaf rosettes develop during the first 2 years, with flowering in the subsequent year, after which the leaf rosette dies (Rønning 1996; Aiken et al. 2007). Maessen et al. (1983) reported that, in a mesic habitat on gravelly soil, its aboveground production comprised 71 % of the total biomass. Once reproductive structures appeared, bulk resources (up to 80 % of aboveground biomass) were allocated to them. The plant is very plastic in its response to the environment, as with other species of the genus Cochlearia (Nordal and Stabbentorp 1990; Morgner and Pettersen 2007). On Svalbard, two main forms can be distinguished. One found on open tundra, gravel and in several different plant communities, has a distinct rosette pressed down towards the ground and a central erect stem with an inflorescence. The latter, growing densely and profusely at the foot of and directly on bird cliffs, has fleshy and long-stemmed leaves which are 2–3 cm in diameter and equal in length or longer then the inflorescence (Rønning 1996). Extensive and luxuriant C. groenlandica occurrence adjacent to large seabird colonies has been observed in many areas of Svalbard (e.g. Odasz 1994; Morgner and Pettersen 2007; authors’ own data).
Sampling and analyses
The study was conducted during July and August of 2005 (soil analyses, guano deposition) and 2006 (C. groenlandica variability, guano deposition). Within the study area, a line transect (~450 m long), consisted of 19 160 × 160 cm sampling plots, was traced down the slope between the colony and the seashore (Fig. 1). Along the transect, more sampling plots were situated in the zone of expected strong impact of the colony where the greatest variation in vegetation was observed than in the coastal area that was less influenced by seabirds and where the vegetation was more homogenous (accurate locations of sampling plots described in Table 1).
Cochlearia groenlandica variability
We collected C. groenlandica individuals from all sampling plots. Starting from the upper left corner of a sampling plot, first 30 non-flowering specimens with a single rosette (without flowers or flowering stems, assuring they were equally no older than 2 years) were harvested from each plot (N = 568 in total, 2 specimens were lost during analyses). If insufficient C. groenlandica individuals were present within a sampling plot, we took plants from the immediate vicinity of the plot (this applied to plots 15–19, which were separated by at least 50 m).
Two parameters were used to characterise individual variability in the population. These were (a) leaf width and (b) above-ground biomass of an individual, chosen because of their high sensitivity to environmental factors (Traiser et al. 2005). The width of a leaf, as it is the photosynthetically active part of the plant, is recognised as a measure of its size (Fonseca et al. 2000; Bragg and Westoby 2002) and thus of the condition of the whole plant. Similarly, biomass shows the productivity of a particular species in changing environmental conditions (Anderson and Polis 1999). For each individual, we measured the maximum width of the three largest leaves (precision 1 mm) and above-ground dry mass of each individual (precision 0.01 g).
We also assessed C. groenlandica cover (%) and total vegetation cover (%) within every second sampling plot (N = 10 in total).
Soil physicochemical analyses
We took samples of soil for physicochemical analyses from every second sampling plot (the same used for vegetation cover analyses, N = 10 in total). We collected three soil samples from three points along the same diagonal of each sampling plot (one from the centre and two from the corners of each quadrat) (N = 30 samples in total). Each sample was taken with a shovel from the soil surface layer (to a depth of ca. 5 cm) and contained about 500 cm3 of soil. At sampling locations with very compact vegetation, we removed and discarded the upper layer of live and dead, poorly decomposed, plant material. We prepared the soil samples for analyses in the laboratory immediately after return from the field. Each sample was divided into four sub-samples, 80 cm3 each, and weighed with electronic scales (precision 0.1 g) before assessment of:
1) Gravimetric water content (%) and organic matter content (%)
Soil sub-samples were ground and dried at 40–60 °C to constant mass. Water content (W) was defined as:
where m w was the mass of water and m s was dry mass of soil (Myślińska 1998).
Soil organic matter content in dried sub-samples was measured at the Institute of Soil Science and Plant Cultivation (State Research Institute), Puławy, Poland, by measuring loss on ignition (Ostrowska et al. 1991; Heiri et al. 2001).
2) Conductivity (μS) and pH
Soil sub-samples were mixed with 160 cm3 of distilled water and mixed by shaking for about 20 min, before being filtered through a sieve (0.5 mm diameter mesh). We measured conductivity and pH in this filtrate using a pH/conductance/salinity metre CPC-401 (Elmetron).
3) Content of nitrogen (NO3 −, NH4 +), potassium (K+) and phosphorus (PO4 3−) (mg kg−1 soil dry mass)
Soil sub-samples were mixed with 200 cm3 of 0.03 N acetic acid. Closed vessels were left for ca. 60 min, being shaken every 10 min. The solution was filtered through a sieve (0.5 mm diameter mesh) and then through a filter paper (MN 640 w, Macherey–Nagel). We analysed the filtrate, diluted with distilled water in the case of sub-samples with very high concentrations, with a photometer LF205 (Slandi) following standard procedures (Cygański 1994).
Changes in soil physical and chemical properties with distance from the colony and with the seabird guano deposition are shown in Zwolicki et al. (2013).
4) Nitrogen isotopic signature δ 15N (‰)
Soil sub-samples were ground and dried at 40–60 °C to constant mass, then sieved through a 0.25 mm mesh to remove stones and large plant debris, and ground with a vibrating mill (LMW-S, Testchem) to a grain size of less than 0.03 mm in diameter. A small amount of each soil sub-sample (1–2 mg, weighed with a microbalance, precision 0.001 mg) was packed into a tin capsule. Nitrogen isotope ratio was determined by a continuous flow mass spectrometer (Thermo Fisher, Delta V Advantage) coupled to an elemental analyser (Thermo Fisher, Flash EA 1112) at University of La Rochelle. Results were expressed in the conventional δ 15N notation, according to the equation:
where R sample was the stable isotope ratio 15N/14N in the analysed sample, and R standard was the stable isotope ratio 15N/14N in the reference material, i.e., atmospheric N2 (Ehleringer and Rundel 1989; Kelly 2000).
Guano deposition
To estimate seabird guano deposition along the studied transect, we placed a black plastic sheet (150 × 150 cm) adjacent to each of 10 sampling plots (the same used for soil and vegetation cover analyses). A digital picture of each sheet was taken every 24 h (Canon PowerShot A95, resolution 5.0 million pixels), on each occasion being cleaned before being re-exposed (9 days total). We estimated the area covered with guano by analysing the pictures with SigmaScan Pro 5.0.0. software. Using an earlier calibration (drying and weighing 10 sheets of known guano covered area and calculating the regression equation (y = 0.008 x, R 2 = 0.7, N = 10; data not shown, Zwolicki et al. 2013), we estimated dry mass of guano deposited over time (g m−2 day−1).
Statistical analysis
To examine relationships between C. groenlandica traits and environmental variables, we used the nonparametric Spearman’s rank correlations because of rather low number of repetition of environmental variables’ measurements (linear Pearson’s correlation coefficient was lower than that of the nonparametric Spearman’s rank correlation in every corresponding relationship) and linear regression models with 95 % confidence intervals as they showed the clearest patterns. We used the median of three values of soil properties from each individual sampling plot. Data of both the C. groenlandica traits and all the environmental variables were logarithmically transformed to reduce variance heterogeneity. As a representative of all the presumably ornithologically modified soil properties (ion, organic matter and water content and conductivity), we used first axis of the principal component analysis (PCA) that explained 75.1 % of those properties total variability and correlated the axis with C. groenlandica traits. During PCA, data were also centred, standardised and divided by standard deviation (ter Braak and Šmilauer 2002). Calculating the regression, we excluded from the analyses the outstanding plots, i.e., plot 1 that was situated directly below the cliff, and showed extreme values of most of the environmental variables and susceptibility for the mechanical damage, and plots 18–19 located at the end of the transect, which might be influenced by the sea (all those plots exist on graphs but have not affected the regression lines). Data were processed using STATISTICA 10.0 (StatSoft, Inc. 2011), MATLAB R2011b (MathWorks, Inc. 2011) and CANOCO 4.55 (ter Braak and Šmilauer 2002).
Results
Cochlearia groenlandica occurred within the entire study area but with noticeable variation in abundance. Directly under the bird cliff, it was the dominant vascular plant, contributing 25 of 30 % of total vegetation cover (including vascular plants, mosses and lichens). Up to 10–15 m downslope, the species contributed 55 of 90 % total cover and, within the next 30 m, 75–80 % of a total of 80–95 % vegetation cover. About 50 m below the colony, the species contributed 50 % of vegetation cover, reducing to only 10 % cover a further 30 m below. From about 125 m to the shore C. groenlandica contributed 3 % cover or less (Table 1).
The size of C. groenlandica specimens differed depending on the location. Both leaf width and individual biomass decreased significantly with distance from the seabird colony (r S = −0.83 and r S = –0.72, respectively, P < 0.001 in both; Table 2; Fig. 2a, b). Closest to the colony, directly beneath the cliff, C. groenlandica individuals weighed 0.959 (±1.430) g on average and their largest leaves were 21.7 (±9.0) mm wide (Table 1). The individual with the widest leaves (46 mm) was collected here. Near the coastline, individuals weighed ca. 0.01 g and had leaves not wider than 3.2 mm. On average, the highest values of leaf width (26.6 ± 10.3 mm) and individual biomass (1.355 ± 1.373 g) were observed within sampling plot 4, i.e., about 10 m below seabird colony. The highest individual biomass (6.500 g) was recorded 15 m from the cliff.
Regression lines (solid) with 95 % confidence intervals (dashed) for leaf width (mm) and individual biomass (g) of C. groenlandica versus distance from the colony (m) (a y = 1.35–0.003x, P < 0.001; b y = –0.27–0.006x, P < 0.001) and guano deposition (g m−2 day−1) (c y = 1.51 + 0.74x, P < 0.001; d y = 0.07 + 1.39x, P < 0.001) gradients. Circle—mean value of 30 C. groenlandica measurements, vertical line—95 % confidence interval for the mean. Grey symbols—data excluded from the regression
Cochlearia groenlandica leaf width and individual biomass strongly correlated with seabird guano deposition (r S = 0.79 and r S = 0.69, respectively, P < 0.001 in each; Table 2; Fig. 2c, d). Relationships with soil δ 15N content were also highly significant (leaf width: r S = 0.48, individual biomass: r S = 0.53, both P < 0.001).
Leaf width and individual biomass of C. groenlandica were positively correlated with conductivity (r S = 0.59 and r S = 0.52, respectively, both P < 0.001; Table 2) and acidity of the soil solution (r S = 0.48 and r S = 0.38, both P < 0.001; Table 2) as well as with phosphate (r S = 0.83 and r S = 0.73, both P < 0.001; Fig. 3a, b), nitrate (r S = 0.74 and r S = 0.69, both P < 0.001; Fig. 3c, d) and potassium (r S = 0.60 and r S = 0.57, both P < 0.001) ion content in soil, but not with ammonium (leaf width: P > 0.05 and individual biomass: r S = −0.13, P < 0.05). We observed a strong negative correlation of C. groenlandica leaf width and individual biomass with soil organic matter (r S = −0.82 and r S = −0.74, both P < 0.001) and water content (r S = −0.80 and r S = −0.71, both P < 0.001). The strongest relationships were recorded between C. groenlandica traits and the first PCA axis that represented all the studied chemical and physical soil properties (leaf width: r S = 0.84 and individual biomass: r S = 0.74, both P < 0.001; Table 2; Fig. 4a, b). Phosphate and nitrate contents were most related to the axis (PO4 3−: r = 0.93 and NO3 −: r = 0.95, both P < 0.001).
Regression lines (solid) with 95 % confidence intervals (dashed) for leaf width (mm) and individual biomass (g) of C. groenlandica with soil content of phosphate (a y = 1.34 + 0.86x, P < 0.001; b y = −5.19 + 1.58x, P < 0.001) and nitrate (c y = –0.19 + 0.71x, P < 0.001; d y = −3.17 + 1.36x, P < 0.001) (mg kg−1 soil dry mass) gradients. Circle—mean value of 30 C. groenlandica measurements, vertical line—95 % confidence interval for the mean. Grey symbols—data excluded from the regression
Regression lines (solid) with 95 % confidence intervals (dashed) for leaf width (mm) and individual biomass (g) of C. groenlandica with the first axis of PCA gradients (a y = 1.08 + 0.48x, P < 0.001; b y = –0.74 + 0.89x, P < 0.001. Circle—mean value of 30 C. groenlandica measurements, vertical line—95 % confidence interval for the mean. Grey symbols—data excluded from the regression
Discussion
This is the first detailed study of C. groenlandica abundance and individual biometric parameter variability along a seabird influenced gradient. In our study area, the species was very abundant adjacent to the colony (where it constituted more than half of the total plant cover), almost completely predominated at 15–45 m from the colony, and then decreased towards the coast. Leaf width of specimens found along the study transect covered the full range of this plant’s leaf size described for Svalbard (Rønning 1996) and the Canadian Arctic Archipelago (Aiken et al. 2007). Along this gradient, from the colony to the sea, C. groenlandica leaf size diminished almost tenfold and individual biomass decreased by around two orders of magnitude (Table 1). Plants achieving the greatest size were found only in the vicinity of seabird colony. Plants occurring below the colony and further down the slope to ca. 150 m distance had large (up to 46 mm width), long-stemmed, succulent leaves and were characteristic for fertile ornithogenic sites as described by Rønning (1996). Individuals of such size and structure were observed in other localities where seabirds abundantly nested, for example, on Fuglefjella (Isfjorden) and southern Bear Island (authors’ own data). Specimens found within lower part of the transect had more regular and low rosettes and smaller leaves. Such C. groenlandica forms may be found in poor habitats (Rønning 1996). Physical and chemical soil properties and plant communities in this part of the transect were similar to that of non-ornithogenic areas (Wojtuń, in Zmudczyńska et al. 2012; Zwolicki et al. 2013). Furthermore, the slightly inclined or completely flat coastal terrace had snow cover for much longer than the slope above (author’s unpubl. data). It is therefore possible that persistent snow cover may have deformed plants and/or shortened the growing season and limited the period of resource acquisition (Sonesson and Callaghan 1991). Nonetheless, no snow-related changes in vegetation morphology were seen within the topographically similar area that was situated along Gnålberget slope away from the colony. Hence, snow cover seems to have relatively low impact on vegetation comparing to seabird manuring. The existence of specialised ecotypes that may use different strategies for survival extends the habitat range occupied by a species, especially in extreme environments, and is often observed in Arctic plants, for instance in Saxifraga oppositifolia (Kume et al. 1999; Crawford 2005; Müller et al. 2012) and Dryas octopetala (McGraw and Antonovics 1983). However, in the case of C. groenlandica, we cannot discriminate between ecotypes with data collected within study area of that size and without transferring specimens along the gradient. Furthermore, the influence of seabirds possibly overlapped with this variability, as leaf width and individual biomass decreased consistently along the transect and with changing soil properties.
Strong relationships observed between C. groenlandica individual traits and soil physical and chemical properties (analysed both one by one and all together in PCA) along the colony-sea axis, and similar correlations between distance from seabird colony and guano deposition versus soil properties, described by Zwolicki et al. (2013), confirm the findings of previous studies that the seabird colony influence appears to be the most important factor modifying habitat conditions in the area (Anderson and Polis 1999; Cocks et al. 1998; Erskine et al. 1998; Wainright et al. 1998; Wait et al. 2005; Stempniewicz et al. 2006; Zmudczyńska et al. 2009, 2012). C. groenlandica individual traits were significantly correlated with guano deposition, soil nitrogen stable isotope ratio and almost all the ornithogenically altered soil parameters (Table 2). The exception was ammonium ion content, which correlated weakly and negatively with C. groenlandica individual biomass and showed no relationship with the plant leaf width. This difference may be due to volatilisation to its gaseous form (NH3), and limited absorption by cation-exchange soil components, and the processes occur where heavy load of excrements is deposited. What is more, lower water content and better aeration of soil close to the colony might promote more intensive nitrification and thus further loss of ammonium (Lindeboom 1984; Staunton Smith and Johnson 1995; Ligeza and Smal 2003). Notably, while C. groenlandica is recognised to be a plant that is characteristic of wetter habitats (Aiken et al. 2007), in the current study its individual biomass and leaf size strongly decreased with higher soil water content, i.e., down the slope (however, a part of the water might be enclosed in bulk undecomposed plant material which means unavailable for C. groenlandica there). This observation indicates that nutrient availability was much more limiting for its growth than water content in this location. Similar observations at Ny-Ålesund (west Spitsbergen) were reported by Wookey et al. (1994), who found no response of Polygonum viviparum vegetative or reproductive structures to the addition of water but significantly increased vegetative and asexual generative development after addition of nutrients. Nevertheless, low contents of nitrogen and phosphorus reduce root hydraulic conductance and thus restrict effective water transport inside a plant and its normal growth (Radin and Matthews 1989; Trubat et al. 2006). This may apply in the lower part of the transect, which was moist but poor in nutrients.
Another Arctic species, alpine saxifrage Saxifraga nivalis, similar to C. groenlandica, increased in abundance and individual size under the influence of the studied colony, but the effect was apparent over a shorter distance. The highest values of S. nivalis individual biomass and leaf width were observed ca. 40 m below the colony and no specimens were found higher on the slope (Zmudczyńska et al. 2008). This might be related to less effective nitrate assimilation resulted from lower nitrate reductase activity (NRA) of S. nivalis (constitutive NRA = 0.81 and fertiliser induced NRA = 1.07 μmol NO2 − g fresh weight−1 h−1) comparing to very high values of this parameter observed for C. groenlandica (constitutive NRA = 3.55 and fertilised NRA = 4.35 μmol NO2 − g fresh weight−1 h−1) (Odasz 1994). The absence of S. nivalis directly below the bird cliff may also be due to mechanical disturbance observed within this site. Small and large rocks commonly become detached from the cliff and roll down the slope, along with remnants thrown out from the nests, continually damaging the ground and harming the vegetation by crushing plant tissues. In such comparison, C. groenlandica appeared to be more resistant to both high fertilisation and mechanical disturbance. It was commonly seen growing directly on rock ledges and in cracks where a large biomass of guano was accumulated. It also colonised the mechanically uncovered grounds as a first species even if did not dominated in the vicinity (authors’ own data).
Our study supports the hypothesis that seabirds have fundamental importance for vegetation growth in the resource-limited Arctic environment. The widespread ruderal species C. groenlandica is capable of growing in poor habitats, but in no other sites than those strongly fertilised by seabirds does it reach anything approaching its maximum size (with leaves up to almost 5 cm wide). Its wide range of occurrence and very well-defined phenotypic response to variation in habitat conditions along the gradient of seabird influence make this plant a useful instrument for further studies, for instance on vegetation limits of tolerance for ornithogenic impact, and resource and biomass allocation strategies in the presence of changing nutrient availability and mechanical disturbance. Highly plastic species as C. groenlandica may also be early responders to aspects of climate change. For instance, increase in temperature causing earlier snow disappearance and subsequently lengthening of the growing season, along with deeper melting of the upper layers of permafrost, may lead to an activity increase in temperature-dependant microorganisms that decompose and mineralise organic matter. This would improve nutrient availability and overall conditions for vegetation growth for species such as C. groenlandica, which was suggested by Chapin and Shaver’ on the basis of 9-year tundra experiments stimulating climate change (Chapin et al. 1995, Chapin and Shaver 1996).
References
Aiken SG, Dallwitz MJ, Consaul LL, McJannet CL, Boles RL, Argus GW, Gillett JM, Scott PJ, Elven R, LeBlanc MC, Gillespie LJ, Brysting AK, Solstad H, Harris JG (2007) Flora of the Canadian Arctic archipelago: descriptions, illustrations, identification, and information retrieval. Cochlearia groenlandica L. NRC Research Press, National Research Council of Canada, Ottawa. http://nature.ca/aaflora/data, accessed on Date 16 Aug 2012
Anderson WB, Polis GA (1999) Nutrient fluxes from water to land: seabirds affect plant nutrient status on Gulf of California islands. Oecologia 118:324–332
Bokhorst S, Huiskes A, Convey P, Aerts R (2007) External nutrient inputs into terrestrial ecosystems of the Falkland Islands and the Maritime Antarctic. Polar Biol 30:1315–1321
Bragg JG, Westoby M (2002) Leaf size and foraging for light in a sclerophyll woodland. Funct Ecol 16:633–639
Callaghan TV (2001) Arctic ecosystems. In: Encyclopedia of life sciences. Wiley, Chichester, http://www.els.net. doi:10.1038/npg.els.0003197. Accessed 29 April 2013
Chapin FS III, Shaver GR (1996) Physiological and growth responses of Arctic plants to a field experiment simulating climatic change. Ecology 77:822–840
Chapin FS III, Shaver GR, Giblin AE, Nadelhoffer KG, Laundre JA (1995) Responses of Arctic tundra to experimental and observed changes in climate. Ecology 76:694–711
Cocks MP, Balfour DA, Stock WD (1998) On the uptake of ornithogenic products by plants on the inland mountains of Dronning Maud Land, Antarctica, using stable isotopes. Polar Biol 20:107–111
Cragg RH, Bardgett RD (2001) How changes in soil faunal diversity and composition within a trophic group influence decomposition processes. Soil Biol Biochem 33:2073–2081
Crawford RMM (2005) Peripheral plant population survival in polar regions. In: Broll G, Keplin B (eds) Mountain ecosystems: studies in treeline ecology. Springer, Berlin, pp 43–76
Cygański A (1994) Chemiczne metody analizy ilościowej. Wydawnictwo Naukowo-Techniczne, Warszawa
Ehleringer JR, Rundel PW (1989) Stable isotopes: history, units, and instrumentation. In: Rundel PW, Rundel JR, Nagy KA (eds) Stable isotopes in ecological research. Springer, New York, pp 1–16
Elvebakk A (1994) A survey of plant associations and alliances from Svalbard. J Veg Sci 5:791–802
Elven R, Murray DF, Razzhivin VY, Yurtsev BA (eds) (2011) Annotated checklist of the panarctic flora (PAF) vascular plants. http://nhm2.uio.no/paf/, accessed 6 June 2013
Erskine PD, Bergstrom DM, Schmidt S, Stewart GR, Craig E, Tweedie CE, Shaw JD (1998) Subantarctic Macquarie Island—a model ecosystem for studying animal-derived nitrogen sources using 15N natural abundance. Oecologia 117:187–193
Eurola S, Hakala AVK (1977) The bird cliff vegetation of Svalbard. Aquilo Ser Bot 15:1–18
Fonseca CR, Overton JM, Collins B, Westoby M (2000) Shifts in trait-combinations along rainfall and phosphorus gradients. J Ecol 88:964–977
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol 25:101–110
Hodkinson ID, Webb NR, Coulson SJ (2002) Primary community assembly on land—the missing stages: why are the heterotrophic organisms always the first. J Ecol 90:569–577
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27
Khoreva MG, Mochalova OA (2009) Plants and birds on the shore of the sea of Okhotsk: balance, crisis, adaptation. Contemp Probl Ecol 2:87–91
Kume A, Nakatsubo T, Bekku Y, Masuzawa T (1999) Ecological significance of different growth forms of purple saxifrage, Saxifraga oppositifolia L., in the high Arctic, Ny-Ålesund, Svalbard. Arct Antarct Alp Res 31:27–33
Ligeza S, Smal H (2003) Accumulation of nutrients in soil affected by perennial colonies of piscivorous birds with reference to biogeochemical cycles of elements. Chemosphere 52:595–602
Lindeboom HJ (1984) The nitrogen pathway in a penguin rookery. Ecology 65:269–277
Madan NJ, Deacon LJ, Robinson CH (2007) Greater nitrogen and/or phosphorus availability increase plant species’ cover and diversity at a high Arctic polar semidesert. Polar Biol 30:559–570
Maessen O, Freedman B, Nams MLN, Svoboda J (1983) Resource allocation in high Arctic vascular plants of differing growth form. Can J Bot 61:1680–1691
MathWorks, Inc. (2011) MATLAB version R2011b. Natick, MA, USA
McGraw JB, Antonovics J (1983) Experimental ecology of Dryas octopetala ecotypes. I. Ecotypic differentiation and life-cycle stages of selection. J Ecol 71:879–897
Meškauskaitė E, Naujalis JR (2006) Structure and dynamics of Saxifraga hirculus L. populations. Ekologija 1:53–60
Morgner E, Pettersen CE (2007) High nutrient levels can compensate for the growth-limiting effect of low temperatures. In: Alsos IG, Körner C, Murray D (eds) Arctic plant ecology: from tundra to polar desert in Svalbard. AB-326 report 2007, UNIS Publication Series, Longyearbyen, pp 73–82
Mulder CPH, Jones H, Kameda K, Palmborg C, Schmidt S, Ellis JC, Orrock JL, Wait DA, Wardle DA, Yang L, Young H, Croll D, Vidal E (2011) Impacts of seabirds on plant and soil properties. In: Mulder CPH, Anderson WB, Towns DR, Bellingham PJ (eds) Seabird islands. Ecology, invasion and restoration. Oxford University Press, New York, pp 135–176
Müller E, Eidesen PB, Ehrich D, Alsos IG (2012) Frequency of local, regional, and long-distance dispersal of diploid and tetraploid Saxifraga oppositifolia (Saxifragaceae) to Arctic glacier forelands. Am J Bot 99:459–471
Myślińska E (1998) Laboratoryjne badania gruntów. Wydawnictwo Naukowe PWN, Warszawa
Niklas K (1996) Differences between Acer saccharum leaves from open and wind-protected sites. Ann Bot 78:61–66
Nordal I, Stabbentorp OE (1990) Morphology and taxonomy of the genus Cochlearia (Brassicaceae) in Northern Scandinavia. Nord J Bot 10:249–263
Odasz AM (1994) Nitrate reductase activity in vegetation below an Arctic bird cliff, Svalbard, Norway. J Veg Sci 5:913–920
Ostrowska A, Gawliński S, Szczubiałka Z (1991) Metody analizy i oceny właściwości gleb i roślin. Instytut Ochrony Środowiska, Warszawa
Pennings SC, Clark CM, Cleland EE, Collins SL, Gough L, Gross KL, Milchunas DG, Suding KN (2005) Do individual plant species show predictable responses to nitrogen addition across multiple experiments? Oikos 110:547–555
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Syst 28:289–316
Radin JW, Matthews MA (1989) Water transport properties of cortical cells in roots of nitrogen- and phosphorus-deficient cotton seedlings. Plant Physiol 89:264–268
Rønning OS (1996) The flora of Svalbard. Norsk Polarinstitut, Oslo
Rubio G, Zhu J, Lynch JP (2003) A critical test of the two prevailing theories of plant response to nutrient availability. Am J Bot 90:143–152
Ryan PG, Watkins BP (1989) The influence of physical factors and ornithogenic products on plant and arthropod abundance at an inland nunatak group in Antarctica. Polar Biol 10:151–160
Semmartin M, Ghersa CM (2006) Intraspecific changes in plant morphology, associated with grazing, and effects on litter quality, carbon and nutrient dynamics during decomposition. Austral Ecol 31:99–105
Smith VR, Froneman PW (2008) Nutrient dynamics in the vicinity of the Prince Edward Islands. In: Chown SL, Froneman PW (eds) The Prince Edward Islands. Land-sea interactions in a changing ecosystem. SUN Press, Stellenbosch, pp 165–179
Smith JL, Mulder CPH, Ellis JC (2011) Seabirds as ecosystems engineers: nutrient inputs and physical disturbance. In: Mulder CPH, Anderson WB, Towns DR, Bellingham PJ (eds) Seabird islands. Ecology, invasion and restoration. Oxford University Press, New York, pp 27–55
Smykla J, Wołek J, Barcikowski A (2007) Zonation of vegetation related to penguin rookeries on King George Island, maritime Antarctic. Arct Antarct Alp Res 39:143–151
Sonesson M, Callaghan TV (1991) Strategies of survival in plants of the Fennoscandian Tundra. Arctic 44:95–105
StatSoft, Inc. Team (2011) STATISTICA (data analysis software system), version 10. www.statsoft.com. Tulsa, Oklahoma: StatSoft, Inc
Staunton Smith J, Johnson CR (1995) Nutrient inputs from seabirds and humans on a populated coral cay. Mar Ecol Prog Ser 124:189–200
Stempniewicz L (1990) Biomass of Dovekie excreta in the vicinity of a breeding colony. Colon Waterbirds 13:62–66
Stempniewicz L (1992) Manuring of tundra near a large colony of seabirds on Svalbard. In: Opaliński KW, Klekowski RZ (eds) Landscape, life world and man in the high Arctic. IE PAN Press, Warszawa, pp 255–269
Stempniewicz L (2005) Keystone species and ecosystem functioning. Seabirds in polar ecosystems. Ecol Quest 6:111–115
Stempniewicz L, Zwolicki A, Iliszko L, Zmudczyńska K, Wojtuń B (2006) Impact of plankton- and fish-eating seabird colonies on the Arctic tundra ecosystem—a comparison. J Ornithol 147(suppl):257–258
Stempniewicz L, Błachowiak-Samołyk K, Węsławski JM (2007) Impact of climate change on zooplankton communities, seabird populations and arctic terrestrial ecosystem—A scenario. Deep-Sea Res 54:2934–2945
Taguchi Y, Wada N (2001) Variations of leaf trait of an alpine shrub Sieversia pentapetala along an altitudinal gradient under a simulated environmental change. Polar Biosci 14:79–87
ter Braak CJF, Šmilauer P (2002) CANOCO reference manual and user’s guide to Canoco for windows: software for canonical community ordination (version 4.5). Microcomputer Power. Ithaca, New York
Theodose TA, Bowman WD (1997) Nutrient availability, plant abundance, and species diversity in two alpine tundra communities. Ecology 78:1861–1872
Thomas DN, Fogg GE, Convey P, Fritsen CH, Gili JM, Gradinger R, Laybourn-Parry J, Reid K, Walton DWH (2008) The biology of polar regions. Oxford University Press, New York
Traiser C, Klotz S, Uhl D, Mosbrugger V (2005) Environmental signals from leaves—a physiognomic analysis of European vegetation. New Phytol 166:465–484
Trubat R, Cortina J, Vilagrosa A (2006) Plant morphology and root hydraulics are altered by nutrient deficiency in Pistacia lentiscus (L.). Trees 20:334–339
Vidal E, Jouventin P, Frenot Y (2003) Contribution of alien and indigenous species to plant-community assemblages near penguin reckeries at Crozet archipelago. Polar Biol 26:432–437
Wainright SC, Haney JC, Kerr CA, Golovkin N, Flint MV (1998) Utilization of nitrogen derived from seabird guano by terrestrial and marine plants at St. Paul, Pribilof Islands, Bering Sea, Alaska. Mar Biol 131:63–71
Wait DA, Aubrey DP, Anderson WB (2005) Seabird guano influences on desert islands: soil chemistry and herbaceous species richness and productivity. J Arid Environ 60:681–695
Wookey PA, Welker JM, Parsons AN, Press MC, Callaghan TV, Lee JA (1994) Differential growth allocation and photosynthetic responses of Polygonum viviparum L. to simulated environmental change at a high Arctic polar semi-desert. Oikos 70:131–139
Zelenskaya LA, Khoreva MG (2006) Growth of the nesting colony of slaty-backed gulls (Larus schistisagus) and plant cover degradation on Shelikan Island (Taui Inlet, the Sea of Okhotsk). Rus J Ecol 37:126–134
Zmudczyńska K, Zwolicki A, Barcikowski M, Iliszko L, Stempniewicz L (2008) Variability of individual biomass and leaf size of Saxifraga nivalis L. along transect between seabirds colony and seashore in Hornsund, Spitsbergen. Ecol Quest 9:37–44
Zmudczyńska K, Zwolicki A, Barcikowski M, Barcikowski A, Stempniewicz L (2009) Spectral characteristics of the Arctic ornithogenic tundra vegetation in Hornsund area, SW Spitsbergen. Pol Polar Res 30:249–262
Zmudczyńska K, Olejniczak I, Zwolicki A, Iliszko L, Convey P, Stempniewicz L (2012) Influence of allochtonous nutrients delivered by colonial seabirds on soil collembolan communities on Spitsbergen. Polar Biol 35:1233–1245
Żółkoś K, Meissner W (2008) The effect of grey heron (Ardea cinerea L.) colony on the surrounding vegetation and the biometrical features of three undergrowth species. Pol J Ecol 56:65–74
Żółkoś K, Meissner W (2010) Influence of cormorant Phalacrocorax carbo colony on biometrical parameters of three-nerved sandwort Moehringia trinervia (Caryophyllaceae) leaves and seeds. Ekológia 29:55–64
Zwolicki A, Zmudczyńska-Skarbek KM, Iliszko L, Stempniewicz L (2013) Guano deposition and nutrient enrichment in the vicinity of planktivorous and piscivorous seabird colonies in Spitsbergen. Polar Biol 36:363–372
Acknowledgments
Special thanks to M. Eng. Krzysztof Muś (University of Gdańsk) for statistical support and to Prof. Peter Convey (British Antarctic Survey, UK) for consultation and linguistic improvements. We also thank Dr. Pierre Richard (University of La Rochelle, France) for help with isotopic analyses. This study was supported by the Polish Ministry of Science and Higher Education (Grant Nos. 1883/P01/2007/32 and IPY/25/2007) and the Polish-Norwegian Research Fund (Grant No. PNRF-234-AI-1/07, ALKEKONGE). The study was performed under the permission of the Governor of Svalbard.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Zmudczyńska-Skarbek, K., Barcikowski, M., Zwolicki, A. et al. Variability of polar scurvygrass Cochlearia groenlandica individual traits along a seabird influenced gradient across Spitsbergen tundra. Polar Biol 36, 1659–1669 (2013). https://doi.org/10.1007/s00300-013-1385-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-013-1385-6