Abstract
Despite a widespread recognition of the role of seabird colonies in the fertilization of nutrient-poor polar terrestrial ecosystems, qualitative and quantitative data documenting any consequential influence on soil invertebrate communities are still lacking. Therefore, we studied community structure and abundance of springtails (Collembola) in ornithogenic tundra near two large seabird colonies in Hornsund, south-west Spitsbergen. We found considerably (5–20×) higher densities and biomass of Collembola in the vicinities of both colonies (the effect extending up to ca. 50 m from the colony edge) than in comparable control areas of tundra not influenced by allochtonous nutrient input. The most common springtails observed in the seabird-influenced areas were Folsomia quadrioculata, Hypogastrura viatica and Megaphorura arctica. The latter species appeared the most resistant to ornithogenic nutrient input and was found commonly closest to the bird colonies. Collembolan abundance decreased with increasing distance from the seabird colonies. However, relationships between collembolan density and specific physicochemical soil parameters and vegetation characteristics were weak. The most important factors were the cover of the nitrophilous green alga Prasiola crispa, total plant biomass and soil solution conductivity, all of which were correlated with distance from the colony and estimated amount by guano deposition. Community composition and abundance of springtails showed no evidence of being influenced of seabird diet, with no differences apparent between communities found in ornithogenic tundra developing in the vicinity of planktivorous and piscivorous seabird colonies. The study provides confirmation of the influence of marine nutrient input by seabirds on soil microfaunal communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arctic terrestrial ecosystems experience severe environmental conditions and generally low nutrient availability, resulting in low primary production. This contrasts strongly with the adjacent marine environment, where high near-surface nutrient concentrations exist due to vertical mixing of water masses, and the predominance of diatoms and the 24 h polar day support high primary production during summer (Cocks et al. 1998; Stempniewicz 2005; Stempniewicz et al. 2007).
Seabirds constitute a major link between these two environments. They feed at sea and breed on land, often in very large colonies. During the summer reproductive season, seabirds deposit considerable amounts of guano, along with feathers, egg shells and carcasses, which locally provide the main sources of nutrients for tundra plants (Lindeboom 1984; Erskine et al. 1998; Ellis 2005; Stempniewicz 2005). For example, during one breeding season in Hornsund, south-west Spitsbergen, little auks (Alle alle) deliver ~60 tonnes of guano dry mass km−2 of colony area (Stempniewicz 1990, 1992). In comparison, over the three month austral summer period on Anchorage Island, maritime Antarctic, guano input contributed ca. 100 mg N m−2, the vast majority of the external nitrogen input at this location (Bokhorst et al. 2007a). Smith and Froneman (2008, Tables 7.1 and 7.2) provide a detailed description of vertebrate (and other marine-derived) nutrient inputs into the terrestrial ecosystems of sub-Antarctic Marion Island. This island has large populations of seals and various birds including penguins, albatrosses, petrels and burrow-nesting species. Total bird guano production is estimated at 4,279 tonnes year−1, of which about 15% by mass is nitrogen. As well as guano, additional sources of nutrients include food remnants and feathers, and dead eggs, chicks and adults deposited close to colonies (Polis et al. 1997; Anderson and Polis 1999; Ellis 2005; Smith and Froneman 2008)—feathers alone account for somewhat less than 10% of the input values of guano on Marion Island (Smith and Froneman 2008, Table 7.2). Higher concentrations of ions crucial for plant growth (NO3 −, NH4 +, PO4 3−, K+), which are present in soil in the vicinity of seabird colonies and even individual nest burrows compared to areas beyond their impact, enhance primary production (Smith 1976; Anderson and Polis 1999; García et al. 2002; Stempniewicz et al. 2006). Substantial guano deposition close to seabird colonies increases nutrient content in the soil (Ryan and Watkins 1989; Wainright et al. 1998; Anderson and Polis 1999; Stempniewicz et al. 2006) and plant biomass (Anderson and Polis 1999; Zmudczyńska et al. 2008), as well as causing changes within plant communities (Vidal et al. 2003; Ellis et al. 2006; Zmudczyńska et al. 2009). This feeds through the local food web, with more intensive use of such areas by invertebrate and vertebrate herbivores (Jakubas et al. 2008), predators and scavengers (Croll et al. 2005).
Soil invertebrates together with microorganisms play a crucial role in decomposition processes and nutrient flows in polar terrestrial ecosystems (e.g. Cragg and Bardgett 2001; Hodkinson et al. 2002; Hopkin 2002; Bokhorst et al. 2007b). Invertebrates perform and regulate mineralization of fresh organic residues, mostly acting in their physical breakdown or as grazers of the microflora, whereas microorganisms are responsible for the chemical degradation of complex molecules. The most important soil invertebrates in the Arctic are springtails, mites, enchytraeids and dipteran larvae, all of which benefit from organic resources (Filser 2002). However, springtails (Collembola) often exceed all other groups both in abundance and species richness (e.g. Uvarov and Byzova 1995; Birkemoe and Leinaas 2000). Typically, collembolan biomass in the tundra ecosystem reaches 150 mg dry mass m−2, a value that is high in comparison with other biomes such as temperate and tropical grasslands and forests (Rusek 1998). Due to feeding on soil microbiota (fungi, bacteria, actinomycetes, algae), in at least some cases selectively (Worland and Lukešová 2000), springtails potentially influence microbial population dynamics and thereby indirectly affect the overlying macroscopic plant community. They also play important roles in mixing and channelling detritus, dispersing microbial propagules and excreting nutrient-rich wastes (Bardgett and Chan 1999).
In spite of widespread recognition of the important role of seabird colonies in enriching otherwise nutrient-poor polar terrestrial ecosystems (Smith 1979; Joly et al. 1987; Ryan and Watkins 1989; Hodkinson et al. 1994, 1998; Byzova et al. 1995; Uvarov and Byzova 1995; Sømme and Birkemoe 1999), qualitative and quantitative data documenting any consequential impacts on their soil invertebrate communities are still limited. The aims of this study were, therefore:
-
(1) To measure species composition, density and biomass of springtails in the vicinity of two seabird colonies and compare them with non-enriched areas.
-
(2) To assess the relationship between the level of guano deposition and collembolan species abundance and community composition along a gradient of guano deposition between the colonies and the coast.
Materials and methods
Study area
The study was conducted on the north coast of Hornsund (south-west Spitsbergen, Svalbard) in an area of tundra between two seabird colonies and the coast:
(1) A colony of little auks (Alle alle, a planktivorous species) situated on Ariekammen mountain (77°00′N 15°31′E) (Transect 1, Fig. 1a). The study area (effectively a ca. 1,000 m transect) ranged from the mountain slope (inclination 25°–45°) consisting of vegetation covered rock debris, where the colony was located, to near-horizontal tundra approaching the seashore.
(2) A mixed colony of Brunnich’s guillemots (Uria lomvia) and black-legged kittiwakes (Rissa tridactyla) (both piscivorous species) situated on Gnålberget cliff (77°01′N 15°52′E) (Transect 2, Fig. 1b). This study area included ca. 500 m between the nesting cliff and seashore, initially with an inclination of 40°–50° directly under the cliff itself and again terminating with the flat plain at the coast.
Both transects were oriented towards (exposed to) the south-east and covered an altitudinal range from sea level to ca. 200 m asl.
Plant communities occurring in these study areas were characteristic of well-fertilized bird-cliff vegetation (Rønning 1996), with vegetation cover typically 90–95% across the transects, excepting the seashore itself and sites directly under the cliff in Transect 2 that were damaged mechanically by falling rocks. Well-developed vascular plants and algal mats predominated over mosses. The zonal character of vegetation along the colony-sea axis was clearly defined (Zmudczyńska et al. 2009). In the centre of the little auk colony (i.e. in the area under greatest ornithogenic impact), a Deschampsia alpina-Prasiola crispa community was present, which gradually changed to vegetation dominated by a Cerastium arcticum-Chrysosplenium tetrandum community towards the edge of the colony, and then to a wet Sanionia uncinata-Straminergon stramineum community on flat tundra. The transect sector closest to the sea was characterized by a Saxifraga oppositifolia-Sanionia uncinata community. On the talus slope beneath the kittiwake and guillemot community (Transect 2), the following plant communities were successively represented down the slope: Cochlearia groenlandica-Prasiola crispa, Cerastium arcticum-Poa alpina, Saxifraga caespitosa, Festuca rubra and, as in Transect 1, Saxifraga oppositifolia-Sanionia uncinata in the zone adjacent to the shore (Wojtuń unpublished data).
We established control areas on topographically similar transects to those on the ornithogenic tundra sites, but that were not impacted by seabirds (Fig. 1, Transect 1C and Transect 2C, respectively). Both control transects covered the same altitudinal range (from sea level to ca. 200 m asl) and had similar aspect (Transect 2C was offset by no more than 25° from Transect 2).Vegetation in these control areas was limited with lower total cover, a higher proportion of mosses, lichens and cyanobacteria, and with no zonation observed. Vegetation along Transect 1C was almost entirely represented by a Sanionia uncinata-Salix polaris community, except immediately adjacent to the coast, where it was again characterized by a Saxifraga oppositifolia-Sanionia uncinata community. Transect 2C included Sanionia uncinata-Straminergon stramineum and Sanionia uncinata-Tortula ruralis communities (Wojtuń unpublished data).
Sampling and analyses
The study was conducted during July and August 2005. Additionally in late July 2006, we replicated guano deposition analyses, and from early June to late August 2006, we measured soil temperature. In both study areas, a line transect was traced down the slope between the colony and seashore consisting of 10–12 sample plots (160 × 160 cm each). The little auk colony covered a large area of the relatively shallow slope and had a less clear-cut boundary than in the case of the cliff-nesting species. Therefore, Transect 1 commenced from the colony centre, while Transect 2 commenced just beneath the nesting cliff; in both cases we considered these points (sampling plot 1) as being the most influenced by seabirds. Subsequent sampling plots were located at increasing distance from the transect starting points as follows: plot 2 (6 m), 3 (15 m), 4 (29 m), 5 (49 m), 6 (79 m), 7 (125 m), 8 (193 m), 9 (296 m), 10 (449 m), 11 (680 m) and plot 12 (1,026 m). Transect 1 (~1,000 m long), therefore, consisted of 12 and Transect 2 (~500 m long) of 10 sample plots. Along each transect, more sampling plots were situated in the zone of expected strong impact of the colony where the greatest variation in vegetation was observed, than in the coastal area that was less influenced by seabirds and where the vegetation was more homogenous.
Similarly, Transect 1C included 12 sampling plots and Transect 2C 11 sampling plots. Thus, all sampling plots along the ornithogenically influenced transects had corresponding plots in the appropriate control transect.
Springtail sampling
We collected three soil cores from three sites along the same diagonal of each sampling plot (one from the centre and two from the corners of each square) (N = 135 cores in total). Samples were taken with a cylindrical probe (diameter 6 cm) from the soil surface (mainly organic) layer containing vegetation covering the area, down to a soil depth of ca. 5 cm. In the laboratory, each sample was placed for 48 h in a modified Tullgren apparatus illuminated with 60 W bulbs (Barton 1995). Extracted animals were preserved in 96% ethanol and identified to species level following Fjellberg (1998, 2007). We recorded and extrapolated their abundance to calculate total density (all species) and densities of particular species (number of individuals m−2). Biomass (g m−2) of the most abundant species (Folsomia quadrioculata and Hypogastrura viatica) was calculated using species specific formulas relating their body mass and length (Dunger 1968).
Since there were distinct differences in springtail density between the upper part of the ornithogenic transects close to the colony and the lower, seashore, part of each transect (Fig. 2), we divided each transect into two sectors. The upper sector consisted of the 5 sample plots adjacent to the colony in Transect 1 and 2 or their equivalents in 1C and 2C. The seashore sector consisted of 5 (in Transect 2) or 6 (all remaining transects) sample plots.
Guano deposition
To estimate seabird guano deposition along the studied transects, we placed a black plastic sheet (150 × 150 cm) adjacent to each sample plot. A digital picture of each sheet was taken every 24 h (Canon PowerShot A95, resolution 5.0 million pixels), on each occasion being cleaned before being re-exposed (Transect 1 and 1C, 6 days in total; Transect 2, 9 days in total; Transect 2C, 1 day). We estimated the area covered with guano by analysing the pictures with SigmaScan Pro 5.0.0. software. Using an earlier calibration (drying and weighing 41 sheets of known guano-covered area, and calculating regression equations separately for planktivores (y = 0.003 x, R 2 = 0.7, N = 31) and piscivores (y = 0.008 x, R 2 = 0.7, N = 10); data not shown, Zwolicki unpublished), we estimated dry mass (DM) of guano deposited over time (g m−2 day−1).
Soil physicochemical analyses
We took samples of soil for physicochemical analyses adjacent to the same collection sites as used for collembolan sampling (N = 123, 12 samples were lost during analyses). Each sample was taken with a shovel from the soil surface layer (to a depth of ca. 5 cm) and contained ca. 500 cm3 of soil. At sampling locations with very compact vegetation, we removed and discarded the upper layer of live and dead, poorly decomposed, plant material. We prepared the soil samples for analyses in the laboratory immediately after return from the field. Each sample was divided into 3 sub-samples, 80 cm3 each, and weighed with electronic scales (precision 0.1 g) before assessment of:
-
(1) Water content per unit dry mass (%)—Soil sub-samples were ground and dried at 40–60°C to constant mass. Water content (W) was defined as: W = (m w m −1s ) 100%, where m w was the mass of water and m s the dry mass of soil (Myślińska 1998)
-
(2) Conductivity and pH—Soil sub-samples were mixed with 160 cm3 of distilled water and mixed by shaking for about 20 min, before being filtered through a sieve (0.5 mm diameter mesh). We measured conductivity (μS) and pH in this filtrate using a pH/conductance/salinity metre CPC-401 (Elmetron)
-
(3) Content of nitrogen (NO3 −, NH4 +), potassium (K+) and phosphorus (PO4 3−)—Soil sub-samples were mixed with 200 cm3 of 0.03 N acetic acid. Closed vessels were left for ca. 60 min, being shaken every 10 min. The solution was filtered through a sieve (0.5 mm diameter mesh) and then through a filter paper (MN 640 w, Macherey–Nagel). We analysed the filtrate, diluted with distilled water in the case of sub-samples with very high concentrations, with a photometer LF205 (Slandi) following standard procedures (Cygański 1994)
-
(4) Soil organic matter content (%) in dried sub-samples was measured at the Institute of Soil Science and Plant Cultivation (State Research Institute), Puławy, Poland, by measuring loss on ignition (Ostrowska et al. 1991; Heiri et al. 2001).
Finally, to provide a description of the soil thermal microenvironment, temperature loggers (Dallas Thermochron iButton DS1921Z-F5, recording range −5°C to +26°C, ± 1°C accuracy, 0.1°C resolution) were buried at a depth of 5 cm next to the centre of each sampling plot in both ornithogenically influenced transects, recording data every hour from early June to late August.
Vegetation analysis
Within all sampling plots along each transect, we identified vascular plants and mosses and measured their percentage contribution to total vegetation cover. Along the second diagonal of each sample plot, we collected five samples of vegetation (20 × 20 cm) for assessment of biomass of live above-ground parts of plants and mosses. The samples were manually divided into vascular plant and moss components, dried to constant mass and weighed. Total biomass, and biomass of the specific vegetation layers, was extrapolated to be expressed per m2.
Statistical analysis
Shannon’s diversity index (Shannon and Weaver 1949) was used to describe springtail species diversity (H′ = Σi (n i N −1) ln (n i N −1), where n i is the number of individuals of species i, and N is the total number of individuals) and evenness (J′ = H′ (ln S)−1, where S is the number of species). Hutcheson’s test was used for diversity comparison between transects (Hutcheson 1970). Springtail density and biomass were compared between transects and between sectors within each transect using the Mann–Whitney’s U test. We analysed relationships between springtail abundance and the various environmental factors using Spearman’s rank correlation. These non-parametric tests were used due to the non-normal distributions of data obtained and the relatively low number of sampling plots. Numerical ordination methods were used to describe total (qualitative and quantitative) variability of springtail communities independent of the environmental variables (linear indirect gradient analysis—Principal Component Analysis (PCA)) and in relation to the environmental factors (linear direct gradient analysis—Redundancy Analysis (RDA)). All species data and most environmental data (except soil water and organic matter content, soil solution pH, guano deposition and distance from the colony) were log-transformed to normalize and smooth their distributions. After RDA, a Monte Carlo permutation test was performed (with 499 permutations) to identify which of the factors significantly influenced the model (ter Braak and Šmilauer 2002). We calculated parametric Pearson’s correlations between selected springtail species as well as between the species and environmental variables from all the sample plots (all transects) when it concerned RDA results as data were log-transformed in that case.
Data were processed using the following software: DIVer 10.o1 (AZB analysis & software (2010). DIVer (diversity indices analysis software), version 10.o1, http://www.azb.com.pl), STATISTICA 8.0 (StatSoft, Inc. (2008). STATISTICA (data analysis software system), version 8.0. http://www.statsoft.com.) and CANOCO 4.5 (ter Braak and Šmilauer 2002).
Results
Species composition, density and biomass of springtails
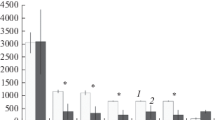
We found seven Collembola taxa in the study area (five identified to species, plus one representative of the genus Isotoma and one of the family Entomobryidae) (Table 1). Four taxa (Folsomia quadrioculata, Hypogastrura viatica, Megaphorura arctica and Isotoma sp.) occurred in all the transects, both ornithogenically influenced and controls. F. quadrioculata and H. viatica were generally found most commonly (in 73–91% of sampling plots of each transect), with the exception of the Transect 2 where H. viatica occurred in only 50% of plots. Tetracanthella arctica occurred in all the transects except for Transect 2C, while Lepidocyrtus lignorum and the unidentified entomobryid were only encountered occasionally, at most in two sample plots within a transect, and only within the control areas. The most abundant species found in the study area were F. quadrioculata, H. viatica and, in sites adjacent to the bird colonies, M. arctica (Fig. 2), whose numbers far exceeded all the other taxa encountered.
The diversity of the springtail community (Shannon’s index), based on the number of individuals obtained in each sampling plot, was lower (Hutcheson’s test; t = 6.73, p < 0.001, df = 887) in both seabird-influenced transects (combined data; H′ = 0.95) compared with the control transects (combined data; H′ = 1.18). The index of evenness based on Shannon’s formula showed no differences between areas, being only slightly lower in the seabird-influenced Transects 1 and 2 (combined data; J′ = 0.59) comparing with the control transects (combined data; J′ = 0.60). When comparing the transects individually, the diversity was lower (t = 9.57, p < 0.001, df = 296) in Transect 1 (H′ = 0.86) than in its control (Transect 1C: H′ = 1.40). The index values obtained within Transect 2 (H′ = 1.00) and its control (Transect 2C: H′ = 0.95) were similar. The index value was significantly higher in Transect 2 than in Transect 1 (t = 7.47, p < 0.001, df = 5,847). The index of evenness showed similar differences between transects, being lower in Transect 1 (J′ = 0.53) than in Transect 1C (J′ = 0.72), but similar between Transect 2 (J′ = 0.62) and Transect 2C (J′ = 0.59).
No consistent linear decrease of Collembola density along the colony-sea axis was observed (Fig. 2). However, there were distinct differences between the upper part of the ornithogenic transects close to the colonies and the lower, seashore, part of each transect. Total springtail density (and that of H. viatica considered separately) in both study areas was much higher adjacent to the colony in comparison with the seashore sectors (p < 0.05, detailed statistics in Table 2). F. quadrioculata density within Transect 2 was similarly higher in the section adjacent to the colony (p < 0.01). No differences were evident in the control areas, except for the case of H. viatica in Transect 2C. Moreover, the seashore sectors of all four transects did not differ significantly in either total collembolan density or that of H. viatica or F. quadrioculata separately (all p > 0.05).
Comparing only the sectors adjacent to the bird colonies, total collembolan density, and that of F. quadrioculata considered separately, were significantly greater in both ornithogenically influenced transects in comparison with their controls (p < 0.05, detailed statistics in Tables 3 and 4). In case of H. viatica, this difference was statistically significant for Transect 1 and its control (p < 0.05). The comparison was not significant for Transect 2, but the overall trend was similar, with the mean and median density values being 4–5 times higher in Transect 2 compared with 2C. We did not find significant differences between the two ornithogenically influenced transects. The frequency of M. arctica was too low to detect any differences between transects, but its occurrence was clearly limited to plots situated close to the bird colonies (Fig. 3).
The biomass of F. quadrioculata and H. viatica showed similar differences between transects as seen with density, with significantly higher values found in sampling plots influenced by both colonies compared with their respective control areas, and no differences between the two colonies (Tables 3, 4).
Environmental factors affecting collembolan abundance
Total collembolan density, and density of H. viatica considered alone, decreased substantially with the distance from the two colonies (r S = −0.45, p < 0.001, N = 66 and r S = −0.41, p < 0.001, N = 66, respectively). Significant correlations were present between springtail density and guano deposition, and most of the environmental factors examined, as well as with plant cover and biomass (Table 5). Soil temperature, measured only within the ornithogenically influenced transects, was not correlated either with total springtail density or with density of individual species.
Principal Component Analysis (PCA), which is independent of environmental variables (i.e. soil and plant characteristics), showed that 59.6% of the total variability of Collembola species composition in the studied plots was explained by two main hypothetical gradients expressed by two axes. Guano deposition used as a sole environmental variable (axis 1 in Redundancy Analysis (RDA)) significantly explained 6.7% (F = 3.44, p = 0.006) of that variability (Fig. 4a). Unidentified factors, represented by the other RDA axes, explained a larger proportion of the species variability: axis 2 = 32.4%, axis 3 = 21.7%, axis 4 = 14.1%. The RDA model testing all environmental variables except for those showing high levels of autocorrelation with each other (inflation factor >20; guano deposition, vascular plant, moss and C. groenlandica cover, phosphorus and both nitrogen ion concentrations), explained 53.7% (F = 1.78, p = 0.002) of springtail community variability. The strongest effect was exerted by Prasiola crispa cover (explaining 11.0% of the total variability, p = 0.002), total plant biomass (9%, p = 0.002) and soil solution conductivity (6%, p = 0.01) (Fig. 4b). Densities of the most abundant species, F. quadrioculata, H. viatica and M. arctica, best fitted the model. F. quadrioculata abundance was significantly positively correlated with total plant biomass (r = 0.56, p < 0.001, N = 44). H. viatica abundance increased with increasing P. crispa cover (r = 0.46, p < 0.01, N = 44) and total plant biomass (r = 0.34, p < 0.05, N = 44), and M. arctica abundance was positively correlated with conductivity (r = 0.39, p < 0.01, N = 44). F. quadrioculata and H. viatica densities were positively correlated with each other (r = 0.32, p < 0.05, N = 44) and were independent of that of M. arctica (p > 0.05, N = 44) (Fig. 4b).
Multivariate analyses of relationships within the springtail community with respect to: a guano deposition and b environmental and biotic factors (RDA, all transects included). Full tip arrows—variables, open tip arrows—springtail species; bold solid lines—significant variables (Monte Carlo permutation test, p < 0.05), regular solid lines—insignificant variables (p > 0.05), dashed lines—supplementary variables (not tested due to high correlations with other variables, not influencing the ordination)
Both P. crispa cover and conductivity were strongly and positively correlated with guano deposition (P. crispa: r = 0.67, conductivity: r = 0.64, p < 0.001, N = 44), and significantly decreased with increasing distance from the seabird colony (P. crispa: r = –0.53, p < 0.05; conductivity: r = –0.54, p < 0.01, N = 22) along the ornithogenically influenced transects (Fig. 4b).
Discussion
Arctic terrestrial ecosystems, generally characterized by chronic nutrient deficiency, can profit from allochtonous matter of marine origin deposited by seabirds, often in large amounts in the vicinity of their colonies. This deposition can facilitate plant and invertebrate community development although, on the other hand, locally excessive tundra fertilization may limit the occurrence of some plants and soil invertebrates to those with the greatest tolerance.
In our study area, two to three Collembola species were encountered commonly and in large numbers, with the remainder being found occasionally and often only within control areas not affected by birds (Table 1; Fig. 2). The two most abundant species, F. quadrioculata and H. viatica, are both widespread in Svalbard (Sømme and Birkemoe 1999; Herzberg et al. 2000). Their densities and biomass, as well as total collembolan density, were 5–20 times higher in parts of both ornithogenically influenced transects compared with their controls (Tables 3, 4; Fig. 3), indicating improved feeding conditions (cf. Hodkinson and Wookey 1999; Bokhorst et al. 2007b). In spite of differences in physicochemical soil parameters and vegetation structure being reported between the ornithogenic tundra developing close to planktivorous and piscivorous seabird colonies (Stempniewicz et al. 2006), we found similar abundance and species composition of springtail communities in the sampling plots influenced by the two representatives of these colony types examined in this study.
Guano fertilization can be excessive in the direct proximity of a bird colony (deposition reaching ca. 1.2–1.5 g m−2 day−1, Zwolicki unpublished data) and such conditions probably only permit the most tolerant species to survive. Springtails existing under these conditions may avoid or limit the associated extreme stresses by, for example, vertical stratification in the soil or the neutralization of high ammonium concentration through increased water uptake (Jensen et al. 2006). They may then benefit from the subsequent tundra plant development providing directly and indirectly an important food resource for them. Such sites here were dominated by M. arctica, a species known for its occurrence close to bird cliffs (Hodkinson et al. 1994), and presumably the collembolan most resistant to high guano deposition.
The density and biomass of springtails did not show a simple linear gradient along the transects from the seabird colonies to the coast, but there were very clear differences between groups of sampling plots close to and remote from the bird colonies. The contrast between these two groups of sample plots indicates that seabird impact on soil invertebrate community composition, while potentially very large, is also spatially limited, here being restricted to within ca. 50 m (i.e. to sample plot 5) of each bird colony (Fig. 2).
Notwithstanding the clear localized colony impact, we found an overall negative relationship between collembolan density and distance from the bird colonies, and a positive correlation with rate of guano deposition and various physicochemical properties of the soil and vegetation characteristics (Table 5). The decrease in springtail density and biomass with increasing distance from the colony distance was clearly not linear (Fig. 2). This probably relates to the most abundant species achieving their optimum occurrence at points below the maximum seabird influence (guano deposition), as suggested by Sømme and Birkemoe (1999). Our data are consistent with previous observations that springtail abundance is not directly dependent on a single factor but is likely related to a suite of complex environmental conditions, such as habitat moisture, temperature, acidity, organic matter content, vegetation cover, soil structure and composition (Hodkinson et al. 1998; Herzberg et al. 2000; Filser 2002; Cassagne et al. 2003; Dollery et al. 2006; Caruso et al. 2007).
Springtail distribution and density often reflect the patchy nature of their habitat, especially in earlier vegetation succession stages (Caruso and Bargagli 2007). Notwithstanding this influence of habitat, microarthropod distributions in polar soils and vegetation profiles are characteristically patchy or uneven, even in otherwise apparently uniform habitats (Usher and Booth 1984, 1986). Distinct springtail assemblages with one or two dominant species may be found in combination with particular plant species or communities and, consequently, be related to specific environmental conditions forming below or near to this vegetation (Herzberg et al. 2000; Ims et al. 2004; Dollery et al. 2006; Caruso and Bargagli 2007). Such relationships between soil properties and vegetation type and invertebrate distribution also impact springtail communities and may involve a form of feedback. Selective habitat choice by Collembola may be connected with food availability, so the litter type or fungal species therein may strongly influence springtail community structure, especially that of microphytophagous and fungivorous species. Moreover, feedback effects may also occur if microarthropod grazing affects decomposition rates and, thereby, the fungal and plant communities themselves (Coulson et al. 2003).
These potentially complex springtail-habitat interactions are partially reflected in the RDA results (Fig. 4). The only factors significantly influencing the springtail community were the cover of the green nitrophilous alga P. crispa, total plant biomass and soil conductivity. P. crispa is known for its growth directly on guano-covered sites close to marine bird and mammal aggregations both in Arctic and Antarctic (Matuła et al. 2007; Smykla et al. 2007). Algal cover and soil conductivity, which is indirectly connected with content of several ions, changed along the seabird colony-sea axis and guano deposition gradient. Herzberg et al. (2000) recognized plant cover as the most important variable explaining density of some collembolans, such as F. quadrioculata. Since in our study area total plant cover was homogeneous and very high across virtually the entire transects (with the exception of plot 1 of Transect 2, directly below the bird cliff, and some plots of Transect 2C), cover values were not included in the RDA. Instead, total plant biomass was used as a measure of vegetation abundance, and appeared to be a significant factor influencing Collembola community composition. Many studies have linked plant biomass and cover increase with seabird presence in the polar regions (e.g. Ellis 2005; Wait et al. 2005; Maron et al. 2006), and our results provide further support to the hypothesis that seabirds impact indirectly on springtail (and other soil invertebrate) community composition and variability, this being mediated through vegetation changes.
Our study has confirmed the locally strong influence of nutrient input from seabird colonies on the springtail communities developing in these areas on High Arctic Svalbard. While the strongest effects were clearly apparent within a relatively short distance—tens of metres—of the colony, it is important also to recognize that springtail distribution shows significant correlations with a range of physical and biological environmental factors and, thus, that a complex suite of these factors and their interactions is likely to underlie these distributions. Furthermore, while impacts of over-fertilization in the immediate vicinity of colonies are likely to be directly limiting to springtails, many of the factors influencing springtail distribution will act indirectly through their consequences for local vegetation and microbial community composition, structure and biomass.
References
Anderson WB, Polis GA (1999) Nutrient fluxes from water to land: seabirds affect plant nutrient status on Gulf of California islands. Oecologia 118:324–332
Bardgett RD, Chan KF (1999) Experimental evidence that soil fauna enhance nutrient mineralization and plant nutrient uptake in montane grassland ecosystems. Soil Biol Biochem 31:1007–1014
Barton TR (1995) A modified technique for extracting live ticks from small soil and litter samples. Exp Appl Acarol 19:357–360
Birkemoe T, Leinaas HP (2000) Effects of temperature on the development of an arctic Collembola (Hypogatrura tullbergi). Funct Ecol 14:693–700
Bokhorst S, Huiskes A, Convey P, Aerts R (2007a) External nutrient inputs into terrestrial ecosystems of the Falkland Islands and the Maritime Antarctic. Polar Biol 30:1315–1321
Bokhorst S, Ronfort C, Huiskes A, Convey P, Aerts R (2007b) Food choice of Antarctic soil arthropods clarified by stable isotope signatures. Polar Biol 30:983–990
Byzova JB, Uvarov AV, Petrova AD (1995) Sesonal changes in communities of soil invertebrates in tundra ecosystem of Hornsund, Spitsbergen. Pol Polar Res 16:245–266
Caruso T, Bargagli R (2007) Assessing abundance and diversity patterns of soil microarthropod assemblages in northern Victoria Land (Antarctica). Polar Biol 30:895–902
Caruso T, Borghini F, Bucci C, Colacevich A, Bargagli R (2007) Modelling local-scale determinants and the probability of microarthropod species occurrence in Antarctic soils. Soil Biol Biochem 39:2949–2956
Cassagne N, Gers C, Gauquelin T (2003) Relationships between Collembola, soil chemistry and humus types in forest stands (France). Biol Fertil Soils 37:355–361
Cocks MP, Balfour DA, Stock WD (1998) On the uptake of ornithogenic products by plants on the inland mountains of Dronning Maud Land, Antarctica, using stable isotopes. Polar Biol 20:107–111
Coulson SJ, Hodkinson ID, Webb NR (2003) Microscale distribution patterns in high Arctic soil microarthropod communities: the influence of plant species within the vegetation mosaic. Ecography 26:801–809
Cragg RH, Bardgett RD (2001) How changes in soil faunal diversity and composition within a trophic group influence decomposition processes. Soil Biol Biochem 33:2073–2081
Croll DA, Maron JL, Estes JA, Danner EM, Byrd GV (2005) Introduced predators transform subarctic islands from grassland to tundra. Science 307:1959–1961
Cygański A (1994) Chemiczne metody analizy ilościowej. Wydawnictwo Naukowo-Techniczne, Warszawa
Dollery R, Hodkinson ID, Jónsdóttir IS (2006) Impact of warming and timing of snow melt on soil microarthropod assemblages associated with Dryas-dominated plant communities on Svalbard. Ecography 29:111–119
Dunger W (1968) Die Entwicklung der Bodenfauna auf rekultivierten Kippen und Halden des Braunkohlentagebaues. Ein Beitrag zur pedozoologischen Standortsdiagnose. Abhandlungen und Berichte des Naturkundemuseums Görlitz 43:1–256
Ellis JC (2005) Marine birds on land: a review of plant biomass, species richness, and community composition in seabird colonies. Plant Ecol 181:227–241
Ellis JC, Farina JM, Witman JD (2006) Nutrient transfer from sea to land: the case of gulls and cormorants in the Gulf of Maine. J Anim Ecol 75:565–574
Erskine PD, Bergstrom DM, Schmidt S, Stewart GR, Craig E, Tweedie CE, Shaw JD (1998) Subantarctic Macquarie Island—a model ecosystem for studying animal-derived nitrogen sources using 15N natural abundance. Oecologia 117:187–193
Filser J (2002) The role of Collembola in carbon and nitrogen cycling in soil. Pedobiologia 46:234–245
Fjellberg A (1998) The Collembola of Fennoscandia and Denmark: Part 1: Poduromorpha. Fauna Entomol Scan 35:1–185
Fjellberg A (2007) The Collembola of Fennoscandia and Denmark: Part 2: Entomobryomorpha and Symphypleona. Fauna Entomol Scan 42:1–264
García LV, Marañón T, Ojeda F, Clemente L, Redondo R (2002) Seagull influence on soil properties, chenopod shrub distribution, and leaf nutrient status in semi-arid Mediterranean islands. Oikos 98:75–86
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolimnol 25:101–110
Herzberg K, Yoccoz NG, Ims RA, Leinaas HP (2000) The effects of spatial habitat configuration on recruitment, growth and population structure in arctic Collembola. Oecologia 124:381–390
Hodkinson ID, Wookey PA (1999) Functional Ecology of soil organisms in tundra ecosystems: towards the future. Appl Soil Ecol 11:111–126
Hodkinson ID, Coulson S, Webb NR, Block W, Strathdee AT, Bale JS (1994) Feeding studies on Onychiurus arcticus (Tullberg) (Collembola: Onychiuridae) on West Spitsbergen. Polar Biol 14:17–19
Hodkinson ID, Webb NR, Bale JS, Block W, Coulson SJ, Strathdee AT (1998) Global change and Arctic ecosystems: conclusions and predictions from experiments with terrestrial invertebrates on Spitsbergen. Arct Alp Res 30:306–313
Hodkinson ID, Webb NR, Coulson SJ (2002) Primary community assembly on land—the missing stages: why are the heterotrophic organisms always the first. J Ecol 90:569–577
Hopkin SP (2002) Collembola. In: Lal R (ed) Encyclopedia of Soil Science. Marcel Dekker, New York, pp 207–210
Hutcheson K (1970) A test for comparing diversities based on the Shannon formula. J Theor Biol 29:151–154
Ims RA, Leinaas HP, Coulson S (2004) Spatial and temporal variation in patch occupancy and population density in a model system of an arctic Collembola species assemblage. Oikos 105:89–104
Jakubas D, Zmudczyńska K, Wojczulanis-Jakubas K, Stempniewicz L (2008) Faeces deposition and numbers of vertebrate herbivores in the vicinity of planktivorous and piscivorous seabird colonies in Hornsund, Spitsbergen. Pol Polar Res 29:45–58
Jensen TC, Leinaas HP, Hessen DO (2006) Age-dependent shift in response to food element composition in Collembola: contrasting effect of dietary nitrogen. Oecologia 149:583–592
Joly Y, Frenot Y, Vernon P (1987) Environmental modifications of a subantarctic peat-bog by the wandering albatross (Diomedea exulans): a preliminary study. Polar Biol 8:61–72
Lindeboom HJ (1984) The nitrogen pathway in a penguin rookery. Ecology 65:269–277
Maron JL, Estes JA, Croll DA, Danner EM, Elmendorf SC, Buckelew SL (2006) An introduced predator alters Aleutian Island plant communities by thwarting nutrient subsidies. Ecol Monogr 76:3–24
Matuła J, Pietryka M, Richter D, Wojtuń B (2007) Cyanoprocaryota and algae of Arctic terrestrial ecosystem in the Hornsund area, Spitsbergen. Pol Polar Res 28:283–315
Myślińska E (1998) Laboratoryjne badania gruntów. Wydawnictwo Naukowe PWN, Warszawa
Ostrowska A, Gawliński S, Szczubiałka Z (1991) Metody analizy i oceny właściwości gleb i roślin. Instytut Ochrony Środowiska, Warszawa
Polis GA, Anderson WB, Holt RD (1997) Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu Rev Ecol Syst 28:289–316
Rønning OS (1996) The flora of Svalbard. Norsk Polarinstitut, Oslo
Rusek J (1998) Biodiversity of Collembola and their functional role in the ecosystem. Biodivers Conserv 7:1207–1219
Ryan PG, Watkins BP (1989) The influence of physical factors and ornithogenic products on plant and arthropod abundance at an inland nunatak group in Antarctica. Polar Biol 10:151–160
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Smith VR (1976) The effect of burrowing species of Procellariidae on the nutrient status of inland tussock grasslands on Marion Island. S Afr J Bot 42:265–272
Smith VR (1979) The influence of seabird manuring on the phosphorus status of Marion Island (Subantarctic) soils. Oecologia 41:123–126
Smith VR, Froneman PW (2008) Nutrient dynamics in the vicinity of the Prince Edward Islands. In: Chown SL, Froneman PW (eds) The Prince Edward Islands. Land-sea interactions in a changing ecosystem. SUN Press, Stellenbosch, pp 165–179
Smykla J, Wołek J, Barcikowski A (2007) Zonation of vegetation related to penguin rookeries on King George Island, maritime Antarctic. Arct Antarct Alp Res 39:143–151
Sømme L, Birkemoe T (1999) Demography and population of Folsomia quadrioculata (Collembola, Isotomidae) on Spitsbergen. Nor J Entomol 46:35–45
Stempniewicz L (1990) Biomass of dovekie excreta in the vicinity of a breeding colony. Colon Waterbirds 13:62–66
Stempniewicz L (1992) Manuring of tundra near a large colony of seabirds on Svalbard. In: Opaliński KW, Klekowski RZ (eds) Landscape, life world and man in the High Arctic. IE PAN Press, Warszawa, pp 255–269
Stempniewicz L (2005) Keystone species and ecosystem functioning. Seabirds in polar ecosystems. Ecol Quest 6:111–115
Stempniewicz L, Zwolicki A, Iliszko L, Zmudczyńska K, Wojtuń B (2006) Impact of plankton- and fish-eating seabird colonies on the Arctic tundra ecosystem—a comparison. J Ornithol 147 (suppl.):257–258
Stempniewicz L, Błachowiak-Samołyk K, Węsławski JM (2007) Impact of climate change on zooplankton communities, seabird populations and arctic terrestrial ecosystem—a scenario. Deep-Sea Res 54:2934–2945
ter Braak CJF, Šmilauer P (2002) CANOCO reference manual and user’s guide to Canoco for Windows: software for Canonical Community Ordination (version 4.5). Microcomputer Power. Ithaca, New York
Usher MB, Booth RG (1984) Arthropod communities in a maritime Antarctic moss-turf habitat: three-dimensional distribution of mites and Collembola. J Anim Ecol 53:427–441
Usher MB, Booth RG (1986) Arthropod communities in a maritime Antarctic moss-turf habitat: multiple scales of pattern in the mites and Collembola. J Anim Ecol 55:155–170
Uvarov AV, Byzova JB (1995) Species diversity and distribution of Collembola in the vicinity of Polish Polar Station, Hornsund area, Spitsbergen. Pol Polar Res 16:233–243
Vidal E, Jouventin P, Frenot Y (2003) Contribution of alien and indigenous species to plant-community assemblages near penguin reckeries at Crozet archipelago. Polar Biol 26:432–437
Wainright SC, Haney JC, Kerr CA, Golovkin N, Flint MV (1998) Utilization of nitrogen derived from seabird guano by terrestrial and marine plants at St. Paul, Pribilof Islands, Bering Sea, Alaska. Mar Biol 131:63–71
Wait DA, Aubrey DP, Anderson WB (2005) Seabird guano influences on desert islands: soil chemistry and herbaceous species richness and productivity. J Arid Environ 60:681–695
Worland MR, Lukešová A (2000) The effect of feeding on specific soil algae on the cold-hardiness of two Antarctic micro-arthropods (Alaskozetes antarcticus and Cryptopygus antarcticus). Polar Biol 23:766–774
Zmudczyńska K, Zwolicki A, Barcikowski M, Iliszko L, Stempniewicz L (2008) Variability of individual biomass and leaf size of Saxifraga nivalis L. along transect between seabirds colony and seashore in Hornsund, Spitsbergen. Ecol Quest 9:37–44
Zmudczyńska K, Zwolicki A, Barcikowski M, Barcikowski A, Stempniewicz L (2009) Spectral characteristics of the Arctic ornithogenic tundra vegetation in Hornsund area, SW Spitsbergen. Pol Polar Res 30:249–262
Acknowledgments
We thank Sławomira Fryderyk and Mateusz Barcikowski (University of Gdańsk) for assistance in data collection and laboratory analyses, and Bronisław Wojtuń (University of Wrocław) and Jan Matuła (Wrocław University of Environmental and Life Sciences) for help in botanical analyses. We are also grateful to three anonymous reviewers and the Editor for their constructive comments on the manuscript. This study was supported by the Polish Ministry of Science and Higher Education (Grant Nos. 1883/P01/2007/32 and IPY/25/2007) and the Polish-Norwegian Research Fund (Grant No. PNRF-234-AI-1/07, ALKEKONGE). The study was performed under the permission of the Governor of Svalbard.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zmudczyńska, K., Olejniczak, I., Zwolicki, A. et al. Influence of allochtonous nutrients delivered by colonial seabirds on soil collembolan communities on Spitsbergen. Polar Biol 35, 1233–1245 (2012). https://doi.org/10.1007/s00300-012-1169-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-012-1169-4