Abstract
Purpose
The objectives of this study were to characterize the population pharmacokinetics of MTX in patients with acute lymphoblastic leukemia (ALL) with ages ranging from 2 to 16 years and to propose a limited sampling strategy to estimate individual pharmacokinetic parameters.
Methods
Seventy-nine children were enrolled in this study; they received 1–4 courses of chemotherapy. MTX was administered at a dose of 5 g/m². MTX population parameters were estimated from 61 patients (231 courses; age range: 2–16 years). The data were analyzed by nonlinear mixed-effect modeling with use of a two-compartment structural model. The interoccasion variability was taken into account in the model. Eighteen additional patients (70 courses) were used to evaluate the predictive performances of the Bayesian approach and to devise a limited sampling strategy.
Results
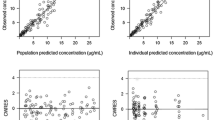
The following population parameters were obtained: total clearance (CL) = 8.8 l/h (inter-individual variability: 43%), initial volume of distribution (V 1) = 17.3 l (48%), k 12 = 0.0225 h−1 (41%), and k 21 = 0.0629 h−1 (24%). The inter-individual variability in the initial volume of distribution was partially explained by the fact that this parameter was weight-dependent. Intercourse variability was limited, with a mean variation of 13.2%. The protocol involving two sampling times, 24 and 48 h after the beginning of infusion, allows precise and accurate determination of individual pharmacokinetic parameters and consequently, it was possible to predict the time at which the MTX concentration reached the predicted threshold (0.2 μM) below which the administration of folinic acid could be stopped.
Conclusion
The results of this study combine the relationships between the pharmacokinetic parameters of MTX and patient covariates that may be useful for dose adjustment, with a convenient sampling procedure that may aid in optimizing pediatric patient care.





Similar content being viewed by others
References
Pui CH, Sandlund JT, Pei D, Rivera GK, Howard SC, Ribeiro RC, Rubnitz JE, Razzouk BI, Hudson MM, Cheng C, Raimondi SC, Behm FG, Downing JR, Relling MV, Evans WE (2003) Results of therapy for acute lymphoblastic leukemia in black and white children. JAMA 290:2001–2007
Pui CH, Reilling MV, Downing JR (2004) Acute lymphoblastic leukemia. N Engl J Med 350:1535–1548
Entz-Werle N, Suciu S, van der Werff ten Bosch J, Vilmer E, Bertrand Y, Benoit Y, Margueritte G, Plouvier E, Boutard P, Vandecruys E, Ferster A, Lutz P, Uyttebroeck A, Hoyoux C, Thyss A, Rialland X, Norton L, Pages MP, Philippe N, Otten J, Behar C, EORTC Children Leukemia Group (2005) Results of 58872 and 58921 trials in acute myeloblastic leukemia and relative value of chemotherapy vs allergenic bone marrow transplantation in first complete remission: the EORTC Children Leukemia Group report. Leukemia 19:2072–2081
Moe PJ, Holen A (2000) High-dose methotrexate in childhood ALL. Paediatr Hematol Oncol 17:615–622
Cohen IJ (2004) Defining the appropriate dosage of folinic acid after high-dose methotrexate for childhood acute lymphatic leukemia that will prevent neurotoxicity without rescuing malignant cells in the central nervous system. J Pediatr Hematol Oncol 26:156–163
Mantadakis E, Cole PD, Kamen BA (2005) High-dose methotrexate in acute lymphoblastic leukemia: where is the evidence for its continued use? Pharmacotherapy 25:748–755
Bleyer WA (1977) Methotrexate: clinical pharmacology. Current status and therapeutic guidelines. Cancer Treat Rev 4:87–101
Sirotnak FM, Moccio DM (1980) Pharmacokinetic basis for differences in methotrexate sensitivity of normal proliferative tissues in the mouse. Cancer Res 40:1230–1234
Relling MV, Fairclough D, Ayers D, Crom WR, Rodman JH, Pui CH, Evans WE (1994) Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol 12:1667–1672
Treon SP, Chabner BA (1996) Concepts in use of high-dose methotrexate. Clin Chem 42:1322–1329
Donelli MG, Zuccheti M, Robatto A, Perlangeli V, D’Incalci M, Masera G, Rossi MR (1995) Pharmacokinetics of HD-MTX in infants, children and adolescents with non-B acute lymphoblastic leukaemia. Med Pediatr Oncol 24:154–159
Crom WR, Glynn AM, Abromowitch M, Pui CH, Dodge R, Evans WE (1986) Use of the automatic interaction detector method to identify patient characteristics related to methotrexate clearance. Clin Pharmacol Ther 39:592–597
Rodman JH, Sunderland M, Kavanagh RL, Ochs J, Yalowich J, Evans WE, Rivera GK (1990) Pharmacokinetics of contineuous infusion of methotrexate and teniposide in paediatric cancer patients. Cancer Res 50:4267–4271
Garre ML, Relling MV, Kalwinski D, Dodge R, Crom WR, Abromowitch M, Pui CH, Evans WE (1987) Pharmacokinetics and toxicity of methotrexate in children with Down syndrome and acute lymphocytic leukaemia. J Pediatr 111:606–612
Borsi JD, Moe PJ (1987) A comparative study of t he pharmacokinetic of methotrexate in a dose range of 0.5 g to 33.6 g/m² in children with acute lymphoblastic leukaemia. Cancer 60:5–13
Lawrence JR, Steele WH, Stuart JF, McNeill CA, McVie JG, Whiting B (1980) Dose dependent methotrexate elimination following bolus intravenous injection. Eur J Clin Pharmacol 17:371–374
Rask C, Albertioni F, Bentzen SM, Schroeder H, Peterson C (1998) Clinical and pharmacokinetic risk factors for high-dose methotrexate-induced toxicity in children with acute lymphoblastic leukaemia. Acta Oncol 37:277–284
Evans WE, Relling MV, Rodman JH, Crom WR, Boyett JM, Pui CH (1998) Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med 338:499–505
Aquerreta I, Aldaz A, Giraldez J, Sierrasesumaga L (2002) Pharmacodynamics of high-dose methotrexate in pediatric patients. Oncology 36:1344–1349
Aquerreta I, Aldaz A, Giraldez J, Sierrasesumaga L (2004) Methotrexate pharmacokinetics and survival in osteosarcoma. Pediatr Blood Cancer 42:52–58
Wall AM, Gajjar A, Link A, Mahmoud H, Pui CH, Relling MV (2000) Individualized methotrexate dosing in children with relapsed acute lymphoblastic leukemia. Leukemia 14:221–225
Crews KR, Liu T, Rodriguez-Galindo C, Tan M, Meyer WH, Panetta JC, Link MP, Daw NC (2004) High-dose methotrexate pharmacokinetics and outcome of children and young adults with osteosarcoma. Cancer 100:1724–1733
Rousseau A, Sabot C, Delepine N, Delepine G, Debord J, Lachatre G, Marquet P (2002) Baysian estimation of methotrexate pharmacokinetic parameters and area under the curve in children and young adults with localised osteosarcoma. Clin Pharmacokinet 41:1095–1104
Odoul F, Le Guellec C, Lamagnere JP, Breilh D, Saux MC, Paintaud G, Autret-Leca E (1999) Prediction of MTX elimination after high dose infusion in children with acute lymphoblastic leukaemia using a population pharmacokinetic approach. Fundam Clin Pharmacol 13:595–604
Vinks AA (2002) The application of population pharmacokinetic modeling to individualized antibiotic therapy. Int J Antimicrob Agents 19:313–322
Du Bois D, Du Bois EF (1989) A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5:303–311
Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A (1976) A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58:259–263
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Beal SL, Sheiner LB (1994) NONMEM user’s guide, University of California at San Francisco, San Francisco, USA
Visual-NM program (1988) Visual-NM user’s manual, version 5.1. Research Development Population Pharmacokinetics, Montpellier, France
Karlsson MO, Scheiner LB (1993) The importance of modelling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm 21:735–750
Al-Banna MK, Kelman AW, Whitng B (1990) Experimental design and efficient parameter estimation in population pharmacokinetics. J Pharmacokinet Biopharm 18:347–360
Drusano GL (1991) Optimal sampling theory and population modelling: application to determination of the influence of the microgravity environment on drug distribution and elimination. J Clin Pharmacol 31:962–967
Endrenyi L (1981) Design of experiments for estimating enzyme and pharmacokinetic experiments. In: Endrenyi L (eds) Kinetic data analysis, design and analysis of enzyme and pharmacokinetic experiments. Plenum Press, New York, 137–167
Hurst AK, Yoshinaga MA, Mitani GH, Foo KA, Jelliffe RW, Harrison EC (1990) Application of a Bayesian method to monitor and adjust vancomycin dosage regimens. Antimicrob Agents Chemother 34:1165–1171
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503–512
Sheiner LB, Beal SL (1981) Evaluation of methods for estimating population pharmacokinetic parameters. II. Biexponential model and experimental pharmacokinetic data. J Pharmacokinet Biopharm 9:635–651
Meibohm B, Läer S, Panetta JC, Barrett J (2005) Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J 7:E475–E487
Note for guidance on clinical investigation of medicinal products in the paediatric population (CPMP/ICH/2711/99). http://www.emea.eu.int/pdfs/human/ich/271199EN.pdf
Patoux A, Bleyzac N, Boddy AV, Doz F, Rubie H, Bastian G, Maire P, Canal P, Chatelut E (2001) Comparison of nonlinear mixed-effect and non-parametric expectation maximisation modelling for Bayesian estimation of carboplatin clearance in children. Eur J Clin Pharmacol 57:297–303
de Hoog M, Schoemaker RC, van den Anker JN, Vinks A (2002) NONMEM and NPEM2 population modeling: a comparison using tobramycin data in neonates. Ther Drug Monit 24:359–365
Skarby T, Jonsson P, Hjorth L, Behrentz M, Bjork O, Forestier E, Jarfelt M, Lonnerholm G, Hoglund P (2003) High-dose methotrexate: on the relationship of methotrexate elimination time vs renal function and serum methotrexate levels in 1164 courses in 264 Swedish children with acute lymphoblastic leukaemia (ALL). Cancer Chemother Pharmacol 51:311–320
Acknowledgments
To the children and their parents, to Maria-Claude Linus-Sorbet for secretary assistance in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00280-007-0550-4
Rights and permissions
About this article
Cite this article
Piard, C., Bressolle, F., Fakhoury, M. et al. A limited sampling strategy to estimate individual pharmacokinetic parameters of methotrexate in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 60, 609–620 (2007). https://doi.org/10.1007/s00280-006-0394-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-006-0394-3