Abstract
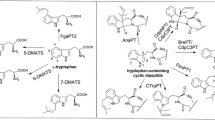
The fungal indole prenyltransferase FtmPT1 is involved in the biosynthesis of fumitremorgins and catalyzes, in the presence of dimethylallyl diphosphate, a predominant regular prenylation of cyclo-l-Trp-l-Pro (brevianamide F) at position C-2 of the indole nucleus. Analysis of the substrate-bound structure of FtmPT1 revealed that brevianamide F forms a hydrogen bond via its carbonyl oxygen in the diketopiperazine moiety with the hydroxyl group of Tyr205 near the center of the prenyltransferase (PT) barrel. In this study, Tyr205 was mutated to 19 other proteinogenic amino acids by one-step site-directed mutagenesis. The obtained mutants were assayed in the presence of dimethylallyl diphosphate with brevianamide F. The enzyme products were isolated on HPLC and their structures were elucidated by NMR and MS analyses. Mutation of Tyr205 to Phe or Met did not change the behavior of FtmPT1 significantly, with regularly C2-prenylated brevianamide F as the predominant product. Interestingly, 15 of the obtained mutants also produced regularly C3-prenylated brevianamide F, with relative yields between 33 and 110 % of those of the regularly C2-prenylated derivatives. Among them, Y205C, Y205L, Y205N, Y205I, and Y205S showed similar brevianamide F consumption. Y205H, Y205Q, Y205V, Y205G, and Y205E showed activities between 47 and 77 % of that of the wild type. These results provide a solid basis for the construction of a brevianamide F regular C3-prenyltransferase by site-directed mutagenesis. Assaying stereoisomers of brevianamide F, cyclo-d-Trp-d-Pro, cyclo-l-Trp-d-Pro, and cyclo-d-Trp-l-Pro, with two selected mutants Y205N and Y205L resulted in the formation of reversely C3-prenylated derivatives as predominant products, being in sharp contrast to their regularly C2- and C3-prenylated derivatives with cyclo-l-Trp-l-Pro.


Similar content being viewed by others
References
Alqahtani N, Porwal SK, James ED, Bis DM, Karty JA, Lane AL, Viswanathan R (2015) Synergism between genome sequencing, tandem mass spectrometry and bio-inspired synthesis reveals insights into nocardioazine B biogenesis. Org Biomol Chem 13:7177–7192
Caballero E, Avendañno C, Menéndez JC (2003) Brief total synthesis of the cell cycle inhibitor tryprostatin B and related preparation of its alanine analogue. J Organomet Chem 68:6944–6951
Fan A, Li S-M (2016) Saturation mutagenesis on Arg244 of the tryptophan C4-prenyltransferase FgaPT2 leads to enhanced catalytic ability and different preferences for tryptophan-containing cyclic dipeptides. Appl Microbiol Biotechnol 100:5389–5399
Fan A, Winkelblech J, Li S-M (2015a) Impacts and perspectives of prenyltransferases of the DMATS superfamily for use in biotechnology. Appl Microbiol Biotechnol 99:7399–7415
Fan A, Zocher G, Stec E, Stehle T, Li S-M (2015b) Site-directed mutagenesis switching a dimethylallyl tryptophan synthase to a specific tyrosine C3-prenylating enzyme. J Biol Chem 290:1364–1373
Grundmann A, Li S-M (2005) Overproduction, purification and characterization of FtmPT1, a brevianamide F prenyltransferase from Aspergillus fumigatus. Microbiology 151:2199–2207
Haynes SW, Gao X, Tang Y, Walsh CT (2013) Complexity generation in fungal peptidyl alkaloid biosynthesis: a two-enzyme pathway to the hexacyclic MDR export pump inhibitor ardeemin. ACS Chem Biol 8:741–748
Heide L (2009) Prenyl transfer to aromatic substrates: genetics and enzymology. Curr Opin Chem Biol 13:171–179
Jost M, Zocher G, Tarcz S, Matuschek M, Xie X, Li S-M, Stehle T (2010) Structure-function analysis of an enzymatic prenyl transfer reaction identifies a reaction chamber with modifiable specificity. J Am Chem Soc 132:17849–17858
Li S-M (2010) Prenylated indole derivatives from fungi: structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat Prod Rep 27:57–78
Li S-M (2011) Genome mining and biosynthesis of fumitremorgin-type alkaloids in ascomycetes. J Anthropol 64:45–49
Metzger U, Schall C, Zocher G, Unsöld I, Stec E, Li S-M, Heide L, Stehle T (2009) The structure of dimethylallyl tryptophan synthase reveals a common architecture of aromatic prenyltransferases in fungi and bacteria. Proc Natl Acad Sci U S A 106:14309–14314
Olafsen T, Kenanove V, Wu AW (2006) Tunable pharmacokinetics: modifying thein vivo half-life of antibodies by directed mutagenesis of the Fc fragment. Nat Protoc 1:2048–2060
Raju R, Piggott AM, Huang XC, Capon RJ (2011) Nocardioazines: a novel bridged diketopiperazine scaffold from a marine-derived bacterium inhibits p-glycoprotein. Org Lett 13:2770–2773
Schuller JM, Zocher G, Liebhold M, Xie X, Stahl M, Li S-M, Stehle T (2012) Structure and catalytic mechanism of a cyclic dipeptide prenyltransferase with broad substrate promiscuity. J Mol Biol 422:87–99
Steffan N, Unsöld IA, Li S-M (2007) Chemoenzymatic synthesis of prenylated indole derivatives by using a 4-dimethylallyltryptophan synthase from Aspergillus fumigatus. Chembiochem 8:1298–1307
Unsöld IA, Li S-M (2005) Overproduction, purification and characterization of FgaPT2, a dimethylallyltryptophan synthase from Aspergillus fumigatus. Microbiology 151:1499–1505
Winkelblech J, Fan A, Li S-M (2015) Prenyltransferases as key enzymes in primary and secondary metabolism. Appl Microbiol Biotechnol 99:7379–7397
Wollinsky B, Ludwig L, Xie X, Li S-M (2012) Breaking the regioselectivity of indole prenyltransferases: identification of regular C3-prenylated hexahydropyrrolo[2,3-b]indoles as side products of the regular C2-prenyltransferase FtmPT1. Org Biomol Chem 10:9262–9270
Woodside AB, Huang Z, Poulter CD (1988) Trisammonium geranyl diphosphate. Org Synth 66:211–215
Yin W-B, Ruan H-L, Westrich L, Grundmann A, Li S-M (2007) CdpNPT, an N-prenyltransferase from Aspergillus fumigatus: overproduction, purification and biochemical characterisation. Chembiochem 8:1154–1161
Yin W-B, Grundmann A, Cheng J, Li S-M (2009) Acetylaszonalenin biosynthesis in Neosartorya fischeri: identification of the biosynthetic gene cluster by genomic mining and functional proof of the genes by biochemical investigation. J Biol Chem 284:100–109
Yin W-B, Xie X-L, Matuschek M, Li S-M (2010) Reconstruction of pyrrolo[2,3-b]indoles carrying an α-configured reverse C3-dimethylallyl moiety by using recombinant enzymes. Org Biomol Chem 8:1133–1141
Yin S, Yu X, Wang Q, Liu XQ, Li S-M (2013) Identification of a brevianamide F reverse prenyltransferase BrePT from Aspergillus versicolor with a broad substrate specificity towards tryptophan-containing cyclic dipeptides. Appl Microbiol Biotechnol 97:1649–1660
Yu X, Zocher G, Xie X, Liebhold M, Schütz S, Stehle T, Li S-M (2013) Catalytic mechanism of stereospecific formation of cis-configured prenylated pyrroloindoline diketopiperazines by indole prenyltransferases. Chem Biol 20:1492–1501
Acknowledgments
We thank Lena Ludwig for synthesis of DMAPP, Rixa Kraut for taking LC–MS analysis, and Stefan Newel for taking NMR spectra. We also thank Sylwia Tarcz for construction of the FtmPT1_Y205F mutant.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was financially supported in part by a grant from the Deutsche Forschungsgemeinschaft (Li844/4-1 to S.-M. L.). Kang Zhou is a recipient of a scholarship from the China Scholarship Council (201308440282).
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 1807 kb)
Rights and permissions
About this article
Cite this article
Zhou, K., Zhao, W., Liu, XQ. et al. Saturation mutagenesis on Tyr205 of the cyclic dipeptide C2-prenyltransferase FtmPT1 results in mutants with strongly increased C3-prenylating activity. Appl Microbiol Biotechnol 100, 9943–9953 (2016). https://doi.org/10.1007/s00253-016-7663-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7663-9