Abstract
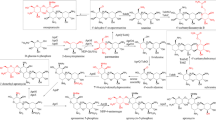
A putative brevianamide F reverse prenyltransferase gene brePT was amplified from Aspergillus versicolor NRRL573 by using primers deduced from its orthologue notF in Aspergillus sp. MF297-2 and overexpressed in Escherichia coli. The soluble His-tagged protein BrePT was purified to near homogeneity and assayed with tryptophan-containing cyclic dipeptides in the presence of dimethylallyl diphosphate. BrePT showed much higher flexibility towards its aromatic substrates than NotF and accepted all of the 14 tested tryptophan-containing cyclic dipeptides. Structure elucidation of the enzyme products by NMR and MS analyses proved unequivocally the highly regiospecific reverse prenylation at C2 of the indole nucleus. K M values of BrePT were determined for its putative substrates brevianamide F and DMAPP at 32 and 98 μM, respectively. Average turnover number (k cat) at 0.4 s−1 was calculated from kinetic data of brevianamide F and DMAPP. K M values in the range of 0.082–2.9 mM and k cat values from 0.003 to 0.15 s−1 were determined for other 11 cyclic dipeptides. Similar to known fungal indole prenyltransferases, BrePT did not accept geranyl or farnesyl diphosphate as prenyl donor for its prenylation.





Similar content being viewed by others
References
Bivin DB, Kubota S, Pearlstein R, Morales MF (1993) On how a myosin tryptophan may be perturbed. Proc Natl Acad Sci U S A 90:6791–6795
Caballero E, Avendañno C, Menéndez JC (2003) Brief total synthesis of the cell cycle inhibitor tryprostatin B and related preparation of its alanine analogue. J Org Chem 68:6944–6951
Cacciatore I, Cocco A, Costa M, Fontana M, Lucente G, Pecci L, Pinnen F (2005) Biochemical properties of new synthetic carnosine analogues containing the residue of 2,3-diaminopropionic acid: the effect of N-acetylation. Amino Acids 28:77–83
Ding Y, Williams RM, Sherman DH (2008) Molecular analysis of a 4-dimethylallyltryptophan synthase from Malbranchea aurantiaca. J Biol Chem 283:16068–16076
Ding Y, Wet JR, Cavalcoli J, Li S, Greshock TJ, Miller KA, Finefield JM, Sunderhaus JD, McAfoos TJ, Tsukamoto S, Williams RM, Sherman DH (2010) Genome-based characterization of two prenylation steps in the assembly of the stephacidin and notoamide anticancer agents in a marine-derived Aspergillus sp. J Am Chem Soc 132:12733–12740
Finefield JM, Greshock TJ, Sherman DH, Tsukamoto S, Williams RM (2011) Notoamide E: biosynthetic incorporation into notoamides C and D in cultures of Aspergillus versicolor NRRL 35600. Tetrahedron Lett 52:1987–1989
Grundmann A, Li S-M (2005) Overproduction, purification and characterization of FtmPT1, a brevianamide F prenyltransferase from Aspergillus fumigatus. Microbiology 151:2199–2207
Guo JP, Tan JL, Wang YL, Wu HY, Zhang CP, Niu XM, Pan WZ, Huang XW, Zhang KQ (2011) Isolation of talathermophilins from the thermophilic fungus Talaromyces thermophilus YM3-4. J Nat Prod 74:2278–2281
Heide L (2009) Prenyl transfer to aromatic substrates: genetics and enzymology. Curr Opin Chem Biol 13:171–179
Kremer A, Li S-M (2008) Potential of a 7-dimethylallyltryptophan synthase as a tool for production of prenylated indole derivatives. Appl Microbiol Biotechnol 79:951–961
Kremer A, Westrich L, Li S-M (2007) A 7-dimethylallyltryptophan synthase from Aspergillus fumigatus: overproduction, purification and biochemical characterization. Microbiology 153:3409–3416
Kuramochi K, Ohnishi K, Fujieda S, Nakajima M, Saitoh Y, Watanabe N, Takeuchi T, Nakazaki A, Sugawara F, Arai T, Kobayashi S (2008) Synthesis and biological activities of neoechinulin A derivatives: new aspects of structure-activity relationships for neoechinulin a. Chem Pharm Bull (Tokyo) 56:1738–1743
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li S-M (2009a) Applications of dimethylallyltryptophan synthases and other indole prenyltransferases for structural modification of natural products. Appl Microbiol Biotechnol 84:631–639
Li S-M (2009b) Evolution of aromatic prenyltransferases in the biosynthesis of indole derivatives. Phytochemistry 70:1746–1757
Li S-M (2010) Prenylated indole derivatives from fungi: structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat Prod Rep 27:57–78
Li G-Y, Yang T, Luo Y-G, Chen X-Z, Fang DM, Zhang G-L (2009) Brevianamide J, a new indole alkaloid dimer from fungus Aspergillus versicolor. Org Lett 11:3714–3717
Liang PH (2009) Reaction kinetics, catalytic mechanisms, conformational changes, and inhibitor design for prenyltransferases. Biochemistry 48:6562–6570
Ritchie R, Saxton JE (1981) Studies on indolic mould metabolites. Total synthesis of l-prolyl-2-methyltryptophan anhydride and deoxybrevianamide E. Tetrahedron 37:4295–4303
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Steffan N, Li S-M (2009) Increasing structure diversity of prenylated diketopiperazine derivatives by using a 4-dimethylallyltryptophan synthase. Arch Microbiol 191:461–466
Steffan N, Unsöld IA, Li S-M (2007) Chemoenzymatic synthesis of prenylated indole derivatives by using a 4-dimethylallyltryptophan synthase from Aspergillus fumigatus. Chem Bio Chem 8:1298–1307
Steffan N, Grundmann A, Yin W-B, Kremer A, Li S-M (2009) Indole prenyltransferases from fungi: a new enzyme group with high potential for the production of prenylated indole derivatives. Curr Med Chem 16:218–231
Tudzynski P, Holter K, Correia T, Arntz C, Grammel N, Keller U (1999) Evidence for an ergot alkaloid gene cluster in Claviceps purpurea. Mol Gen Genet 261:133–141
Wallwey C, Li S-M (2011) Ergot alkaloids: structure diversity, biosynthetic gene clusters and functional proof of biosynthetic genes. Nat Prod Rep 28:496–510
Williams RM, Stocking EM, Sanz-Cervera JF (2000) Biosynthesis of prenylated alkaloids derived from tryptophan. Topics Curr Chem 209:97–173
Woodside AB, Huang Z, Poulter CD (1988) Triammonium germanyl diphosphate. Org Synth 66:211–215
Yazaki K, Sasaki K, Tsurumaru Y (2009) Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry 70:1739–1745
Yin W-B, Grundmann A, Cheng J, Li S-M (2009) Acetylaszonalenin biosynthesis in Neosartorya fischeri: identification of the biosynthetic gene cluster by genomic mining and functional proof of the genes by biochemical investigation. J Biol Chem 284:100–109
Yin W-B, Yu X, Xie X-L, Li S-M (2010) Preparation of pyrrolo[2,3-b]indoles carrying a ß-configured reverse C3-dimethylallyl moiety by using a recombinant prenyltransferase CdpC3PT. Org Biomol Chem 8:2430–2438
Yu X, Liu Y, Xie X, Zheng X-D, Li S-M (2012) Biochemical characterization of indole prenyltransferases: filling the last gap of prenylation positions by a 5-dimethylallyl tryptophansynthase from Aspergillus clavatus. J Biol Chem 287:1371–1380
Zou H-X, Xie X-L, Linne U, Zheng X-D, Li S-M (2010) Simultaneous C7- and N1-prenylation of cyclo-l-Trp-l-Trp catalyzed by a prenyltransferase from Aspergillus oryzae. Org Biomol Chem 8:3037–3044
Acknowledgments
This work was financially supported in part by grants from the Deutsche Forschungsgemeinschaft (Li844/1-3 to S.-M. Li) and China Natural Science Foundation (31070067 to X.-Q. Liu). Xia Yu is a recipient of a fellowship from China Scholarship Council.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Suqin Yin and Xia Yu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1447 kb)
Rights and permissions
About this article
Cite this article
Yin, S., Yu, X., Wang, Q. et al. Identification of a brevianamide F reverse prenyltransferase BrePT from Aspergillus versicolor with a broad substrate specificity towards tryptophan-containing cyclic dipeptides. Appl Microbiol Biotechnol 97, 1649–1660 (2013). https://doi.org/10.1007/s00253-012-4130-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4130-0