Abstract
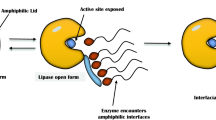
Lipases, triacylglycerol hydrolases, are an important group of biotechnologically relevant enzymes and they find immense applications in food, dairy, detergent and pharmaceutical industries. Lipases are by and large produced from microbes and specifically bacterial lipases play a vital role in commercial ventures. Some important lipase-producing bacterial genera include Bacillus, Pseudomonas and Burkholderia. Lipases are generally produced on lipidic carbon, such as oils, fatty acids, glycerol or tweens in the presence of an organic nitrogen source. Bacterial lipases are mostly extracellular and are produced by submerged fermentation. The enzyme is most commonly purified by hydrophobic interaction chromatography, in addition to some modern approaches such as reverse micellar and aqueous two-phase systems. Most lipases can act in a wide range of pH and temperature, though alkaline bacterial lipases are more common. Lipases are serine hydrolases and have high stability in organic solvents. Besides these, some lipases exhibit chemo-, regio- and enantioselectivity. The latest trend in lipase research is the development of novel and improved lipases through molecular approaches such as directed evolution and exploring natural communities by the metagenomic approach.
Similar content being viewed by others
References
Abdel-Fattah YR (2002) Optimization of thermostable lipase production from a thermophilic Geobacillus sp. using Box–Behnken experimental design. Biotechnol Lett 24:1217–1222
Abdou AM (2003) Purification and partial characterization of psychrotrophic Serratia marcescens lipase. J Dairy Sci 86:127–132
Aires-Barros MR, Taipa MA, Cabral JMS (1994) Isolation and purification of lipases. In: Wooley P, Petersen SB (eds) Lipases—their structure, biochemistry and application. Cambridge University Press, Cambridge, pp 243–270
Albertsson PA, Johansson G, Tjerneld F (1990) Aqueous two-phase separations. In: Asenjo JA (ed) Separation processes in biotechnology. Dekker, New York, pp 287–317
Andersson RE, Hedlund GB, Jensson V (1979) Thermal inactivation of a heat-resistant lipase produced by the psychrotrophic bacterium Pseudomonas fluorescens. J Dairy Sci 62:361–367
Angultra J, Rodrigue Z, Aparicio LB, Naharrao G (1993) Purification, gene cloning, amino acid sequence analysis and expression of an extracellular lipase from an Aeromonas hydrophila human isolate. Appl Environ Microbiol 59:2411–2417
Antonian E (1988) Recent advances in the purification, characterization and structure determination of lipases. Lipids 23:1101–1106
Arnold FH (1996) Directed evolution: creating biocatalysts for the future. Chem Eng Sci 51:5091–5102
Arpigny JL, Jaeger K-E (1999) Bacterial lipolytic enzymes: classification and properties. Biochem J 343:177–183
Bandmann N, Collet E, Leijen J, Uhlen M, Veide A, Nygren PA (2000) Genetic engineering of the Fusarium solani pisi lipase cutinase for enhanced partitioning in PEG-phosphate aqueous two-phase systems. J Biotechnol 79:161–172
Barbaro SE, Trevors JT, Inniss WE (2001) Effects of low temperature, cold shock, and various carbon sources on esterase and lipase activities and exopolysaccharide production by a psychrotrophic Acinetobacter sp. Can J Microbiol 47:194–205
Beisson F, Tiss A, Rivière C, Verger R (2000) Methods for lipase detection and assay: a critical review. Eur J Lipid Sci Technol 133–153
Bell PJ, Sunna A, Gibbs MD, Curach NC, Nevalainen H, Berquist PL (2002) Prospecting for novel lipase genes using PCR. Microbiology 148:2283–2291
Bezborodov AM, Davranov KD, Akhmedova A (1985) Lipase inhibitor in Rhizopus microsporus cultures. In: Kulaev IS, Dawes EA, Tempest DW (eds) FEMS Symposium 23. Academic Press, London, pp 145–149
Bompensieri S, Gonzalez R, Kok R, Miranda MV, Nutgeren-Eoodzant I, Hellingwerf KJ, Cascone O, Nudel BC (1996) Purification of a lipase from Acinetobacter calcoaceticus AAC323-1 by hydrophobic-interaction methods. Biotechnol Appl Biochem 23:77–81
Borgstrom B, Donner J (1976) Interactions of pancreatic lipase with bile salts and dodecylsulfate. J Lipid Res 17:491–497
Bradoo S, Saxena RK, Gupta R (1999) Two acidothermotolerant lipases from new variants of Bacillus spp. World J Microbiol Biotechnol 15:87–91
Bradoo S, Rathi P, Saxena RK, Gupta R (2002) Microwave-assisted rapid characterization of lipase selectivities. J Biochem Biophys Methods 51:115–120
Brune AK, Gotz F (1992) Degradation of lipids by bacterial lipases. In: Winkelman G (ed) Microbial degradation of natural products. VCH, Weinhein, pp 243–266
Cambau B, Klibanov AM (1984) Preparative production of optically active esters and alcohols wing esterase-catalyzed stereospecific trans-esterification in organic media. J Am Chem Soc 106:2687–2692
Castro MJM, Cabral JMS (1988) Reversed micelles in biotechnological processes. Biotechol Adv 6:151–167
Davranov K (1994) Microbial lipases in biotechnology (review). Appl Biochem Microbiol 30:527–534
Dharmsthiti S, Kuhasuntisuk B (1998) Lipase from Pseudomonas aeruginosa LP602: biochemical properties and application for wastewater treatment. J Ind Microbiol Biotechnol 21:75–80
Dharmsthiti S, Luchai S (1999) Production, purification and characterization of thermophilic lipase from Bacillus sp. THL027. FEMS Microbiol Lett 179:241–246
Dharmsthiti S, Pratuangdejkul J, Theeragool GT, Luchai S (1998) Lipase activity and gene cloning of Acinetobacter calcoaceticus LP009. J Gen Appl Microbiol 44:139–145
Dong H, Gao S, Han S, Cao S (1999) Purification and characterization of a Pseudomonas sp. lipase and its properties in non-aqueous media. Appl Microbiol Biotechnol 30:251–256
Dunhaupt A, Lang S, Wagner F (1991) Properties and partial purification of a Pseudomonas cepacia lipase. In: Alberghina L, Schmid RD, Verger R (eds) Lipases: structure, mechanism and genetic engineering. (GBF monographs, vol 16) VCH, Weinheim, pp 389–392
Finkelstein AE, Strawich ES, Sonnino S (1970) Characterization and partial purification of a lipase from Pseudomonas aeruginosa. Biochim Biophys Acta 206:380–391
Gargouri Y, Julian R, Sugihara A, Verger R, Sarda L (1984) Inhibition of pancreatic and microbial lipase by proteins. Biochim Biophys Acta 795:326–331
Ghanem EH, Al-Sayeed HA, Saleh KM (2000) An alkalophilic thermostable lipase produced by a new isolate of Bacillus alcalophilus. World J Microbiol Biotechnol 16:459–464
Ghosh PK, Saxena RK, Gupta R, Yadav RP, Davidson WS (1996) Microbial lipases: production and applications. Sci Prog 79:119–157
Gilbert EJ, Drozd JW, Jones CW (1991a) Physiological regulation and optimization of lipase activity in Pseudomonas aeruginosa EF2. J Gen Microbiol 137:2215–2221
Gilbert EJ, Cornish A, Jones CW (1991b) Purification and properties of extracellular lipase from Pseudomonas aeruginosa EF2. J Gen Microbiol 137:2223–2229
Godfrey T, West S (1996) The application of enzymes in industry. In: Godfrey T, Reichelt J (eds) Industrial enzymology, 2nd edn. The Nature Press, New York, p. 512
Godtfredsen SE (1990) Microbial lipases. In: Fogarty WM, Kelly ET (eds) Microbial enzymes and biotechnology, Elsevier, Amsterdam, pp 255–274
Gupta JK, Soni SK (2000) Industrial uses of enzymes. J Punjab Acad Sci 2:75–80
Gupta R, Bradoo S, Saxena RK (1999) Aqueous two-phase systems: an attractive technology for downstream processing of biomolecules. Curr Sci 77:520–523
Gupta R, Rathi P, Gupta N, Bradoo S (2003) Lipase assays for conventional and molecular screening: an overview. Biotechnol Appl Biochem 37:63–71
Harlow E, Lane D (1988) Antibiodies. Cold Spring Harbor Publications, Cold Spring Harbor, N.Y.
Hassing GS (1971) Partial purification and some properties of a lipase from Corynebacterium acne. Biochem Biophys Acta 242:331
Henne A, Schmitez RA, Bomeke M, Gottschalk G, Daniel R (2000) Screening of environmental DNA libraries for the presence of genes conferring lipolytic activity on Escherichia coli. Appl Environ Microbiol 66:3113–3116
Hirohara H, Mitsuda S, Ando E, Komaki R (1985) Enzymatic preparation of optically active alcohols related to synthetic pyrethroid insecticides. Stud Org Chem 22:119–134
Hong MC, Chang MC (1998) Purification and characterization of an alkaline lipase from a newly isolated Acinetobacter radioresistens CMC-1. Biotechnol Lett 20:1027–1029
Horiuti Y, Imamura S (1977) Purification of lipase from Chromobacterium viscosum by chromatography on palmitoyl cellulose. J Biochem 81:1639–1649
Ihara F, Kageyama Y, Hirata M, Nishira T, Yamada Y (1991) Purification, characterization and molecular cloning of lactonising lipase from Pseudomonas species. J Biol Chem 266:18135–18140
Iizumi T, Nakamura K, Fukase T (1990) Purification and characterization of a thermostable lipase from newly isolated Pseudomonas sp. KWI-56. Agric Biol Chem 545:1253–1258
Imamura S, Kitaura S (2000) Purification and characterization of a monoacylglycerol lipase from the moderately thermophilic Bacillus sp. H-257. J Biochem 127:419–425
Jaeger K-E, Eggert T (2002) Lipases for biotechnology. Curr Opin Biotechnol 13:390–397
Jaeger K-E, Reetz MT (1998) Microbial lipases form versatile tools for biotechnology. Trends Biotechnol 16:396–403
Jaeger K-E, Reetz MT (2000) Directed evolution of enantioselective enzymes for organic chemistry. Curr Opin Chem Biol 4:68–73
Jaeger K-E, Ransac S, Dijkstra BW, Colson C, Heuvel M van, Misset O (1994) Bacterial lipases. FEMS Microbiol Rev 15:29–63
Jaeger K-E, Dijkstra BW, Reetz MT (1999) Bacterial biocatalysts: molecular biology, three-dimensional structures and biotechnological applications of lipases. Annu Rev Microbiol 53:315–351
Jaeger K-E, Eggert T, Eipper A, Reetz MT (2001) Directed evolution and the creation of enantioselective biocatalysts. Appl Microbiol Biotechnol 55:519–530
Jose J, Kurup GM (1999) Purification and characterization of an extracellular lipase from a newly isolated thermophilic Bacillus pumilus. Ind J Exp Biol 37:1213–1217
Kalil SJ, Maugeri F, Rodrigues MI (2000) Response surface analysis and simulation as a tool for bioprocess design and optimization. Process Biochem 35:539–550
Kanwar L, Goswami P (2002) Isolation of a Pseudomonas lipase produced in pure hydrocarbon substrate and its applications in the synthesis of isoamyl acetate using membrane-immobilized lipase. Enzyme Microb Technol 31:727–735
Kanwar L, Gogoi BK, Goswami P (2002) Production of a Pseudomonas lipase in n-alkane substrate and its isolation using an improved ammonium sulfate precipitation technique. Bioresour Technol 84:207–211
Kar M, Ray L, Chattopadhyay P (1996) Isolation and identification of alkaline thermostable lipase producing microorganism and some properties of crude enzyme. Ind J Exp Biol 34:535–538
Kazlauskas RJ, Bornscheuer U (1998) Biotransformations with lipases. In: Rehm HJ, Reeds G (eds) Biotechnology, vol 8a. Wiley–VCH, New York, pp 37–192
Kennedy M, Krouse D (1999) Strategies for improving fermentation medium performance: a review. J Ind Microbiol Biotechnol 23:456–475
Khyami-Horani H (1996) Thermotolerant strain of Bacillus licheniformis producing lipase. World J Microbiol Biotechnol 12:399–401
Kim E-K, Sung M-H, Kim H-M, Oh T-K (1994) Occurrence of thermostable lipase in thermophilic Bacillus sp. strain 398. Biosci Biotechnol Biochem 58:961–962
Kim HK, Choi HJ, Kim MH, Sohn CB, Oh TK (2002) Expression and characterization of Ca(2+)-independent lipase from Bacillus pumilus B26. Biochim Biophys Acta 1583:205–212
Kim KK, Song HK, Shin DH, Hwang KY, Suh DW (1997) The crystal structure of a triglycerol lipase from Pseudomonas cepacia reveals a highly open confirmation in the absence of bound inhibitor. Structure 5:173–185
Kim M-H, Kim H-K, Lee J-K, Park S-Y, Oh TK (2000) Thermostable lipase of Bacillus stearothermophilus: high-level production, purification, and calcium-dependent thermostability. Biosci Biotechnol Biochem 64:280–286
Kim SS, Kim EK, Rhee JS (1996) Effects of growth rate on the production of Pseudomonas fluorescens lipase during the fed-batch cultivation of Escherichia coli. Biotechnol Prog 12:718–722
Kirk O, Borchert TV, Fuglsang CC (2002) Industrial enzyme applications. Curr Opin Biotechnol 13:345–351
Kojima Y, Yokoe M, Mase T (1994) Purification and characterization of an alkaline lipase from Pseudomonas fluorescens AK 102. Biosci Biotechnol Biochem 58:1564–1568
Kordel M, Hofmann B, Schaumburg D, Schmid RD (1991) Extracellular lipase of Pseudomonas sp. strain ATCC 21808: purification, characterization, crystallization and preliminary X-ray diffraction data. J Bacteriol 173:4836–4841
Koritala S, Hesseltine CW, Pryde EH, Mounts TL (1987) Biochemical modification of fats by microorganisms: a preliminary study. J Am Oil Chem Soc 64:509–513
Kulkarni N, Gadre RV (1999) A novel alkaline, thermostable, protease-free lipase from Pseudomonas sp. Biotechnol Lett 21:897–899
Kulkarni N, Gadre RV (2002) Production and properties of an alkaline, thermophilic lipase from Pseudomonas fluorescens NS2W. J Ind Food Microbiol 28:344–348
Lanser AC, Manthey LK, Hou CT (2002) Regioselectivity of new bacterial lipases determined by hydrolysis of triolein. Curr Microbiol 44:336–340
Lavayre J, Verrier J, Baratti J (1982) Stereospecific hydrolysis by soluble and immobilized lipases. Biotechnol Bioeng 24:2175–2188
Lee O-W, Koh Y-S, Kim K-J, Kim B-C, Choi H-J, Kim D-S, Suhartono MT, Pyun Y-R (1999) Isolation and characterization of a thermophilic lipase from Bacillus thermoleovorans ID-1. FEMS Microbiol Lett 179:393–400
Lee SY, Rhee JS (1993) Production and partial purification of a lipase from Pseudomonas putida 3SK. Enzyme Microb Technol 15:617–623
Lee SY, Rhee JS (1994) Hydrolysis of triglyceride by the whole cell of Pseudomonas putida 3SK in two-phase batch and continuous reactor systems. Biocatal Bioeng 44:437–443
Lengsfeld DH, Wolfer H (1988) Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipstatin. Biochem J 256:357–361
Lesuisse E, Schanck K, Colson C (1993) Purification and preliminary characterization of the extracellular lipase of Bacillus subtilis 168, an extremely basic pH-tolerant enzyme. Eur J Biochem 216:155–160
Liese A, Seelbach K, Wandrey C (2001) Industrial biotransformations. Wiley–VCH, Weinheim
Lin SF, Chiou CM, Yeh CM, Tsai YC (1996) Purification and partial characterization of an alkaline lipase from Pseudomonas pseudoalcaligenes F-111. Appl Environ Microbiol 62:1093–1095
Litthauer D, Ginster A, Skein EVE (2002) Pseudomonas luteola lipase: a new member of the 320-residue Pseudomonas lipase family. Enzyme Microb Technol 30:209–215
Liu IL, Tsai SW (2003) Improvements in lipase production and recovery form Acinetobacter radioresistens in presence of polypropylene powders filled with carbon sources. Appl Biochem Biotechnol 104:129–140
Lolis E, Petsko G (1990) Transition state analogues in protein crystallography probes of the structural source of enzyme catalysis. Annu Rev Biochem 59:597–630
Lopes Mde F, Leitao AL, Regalla M, Marques JJ, Carrondo MJ, Crespo MT (2002) Characterization of a highly thermostable extracellular lipase from Lactobacillus plantarum. Int J Food Microbiol 76:107–115
Lorenz P, Liebeton K, Niehaus F, Eck J (2002) Screening for novel enzymes for biocatalytic processes: accessing the metagenome as a resource of novel functional sequence space. Curr Opin Biotechnol 13:572–577
Lotrakul P, Dharmsthiti S (1997) Lipase production by Aeromonas sobria LP004 in a medium containing whey and soyabean meal. World J Microbiol Biotechnol 13:163–166
Lotti M, Monticelli S, Montesinos JL, Brocca S, Valero F, Lafuente J (1998) Physiological control on the expression and secretion of Candida rugosa lipase. Chem Phys Lipids 93:143–148
Macrae AR, Hammond RC (1985) Present and future applications of lipases. Biotech Genet Eng Rev 3:193–217
Mahler GF, Kok RG, Cordenons A, Hellingwerf KJ, Nudel BC (2000) Effects of carbon sources on extracellular lipase production and lipA transcription in Acinetobacter calcoaceticus. J Ind Microbiol Biotechnol 24:25–30
Matsumae H, Furui M, Shibatani T (1993) Lipase-catalyzed asymmetric hydrolysis of 3-phenylglycidic acid ester, the key intermediate in the synthesis of diltiazem hydrochloride. J Ferment Bioeng 75:93–98
Matsumae H, Furul M, Shibatani T, Tosa T (1994) Production of optically active 3-phenylglycidic acid ester by the lipase from Serratia marcescens on a hollow-fiber membrane reactor. J Ferment Bioeng 78:59–63
Misset O, Gerritse G, Jaeger K-E, Winkler U, Colson C, Schanchk K, Lesuisse E, Dartois Y, Blaawoo M, Ransac S, Dijkstra BW (1994) The structure function relationship of the lipases from Pseudomonas aeruginosa and Bacillus subtilis. Protein Eng 7:523–529
Mitsuda S, Umemura T, Hirihara H (1988) Preparation of an optically pure secondary alcohols of synthetic pyrethroids using microbial lipases. Appl Microbiol Biotechnol 29:310–315
Muralidhar RV, Chirumamilla RR, Marchant R, Ramachandran VN, Ward OP, Nigam P (2002) Understanding lipase stereoselectivity. World J Microbiol Biotechnol 18:81–97
Muraoka T, Ando T, Okuda H (1982). Purification and properties of a novel lipase from Staphylococcus aureus 226. J Biochem 92:1933–1939
Nardini M, Dijkstra BW (1999) α/β Hydrolase fold enzymes: the family keeps growing. Curr Opin Struct Biol 9:732–737
Nashif SA, Nelson FE (1953) The lipase of Pseudomonas fragi II: factors affecting lipase production. J Dairy Sci 36:471–480
Nawani N, Kaur J (2000) Purification, characterization and thermostability of a lipase from a thermophilic Bacillus sp. J33. Mol Cell Biochem 206:91–96
Odera M, Takeeuchi K, Tohe A (1986) Molecular cloning of lipase genes from Alcaligenes denitrificans and their expression in Escherichia coli. J Ferment Technol 64:363–371
Oh B-C, Kim H-K, Lee J-K, Kang S-C, Oh T-K (1999) Staphylococcus haemolyticus lipase: biochemical properties, substrate specificity and gene cloning. FEMS Microbiol Lett 179:385–392
Pabai F, Kermasha S, Morin A (1995) Interesterification of butter fat by partially purified extracellular lipases from Pseudomonas putida, Aspergillus niger and Rhizopus oryzae. World J Microbiol Biotechnol 11:669–677
Pabai F, Kermasha S, Morin A (1996) Use of continuous culture to screen for lipase-producing microorganisms and interesterification of butterfat by lipase isolates. Can J Microbiol 42:446–452
Paiva AL, Balcão VM, Malacta FX (2000) Review: kinetics and mechanisms of reactions catalyzed by immobilized lipases. Enzyme Microbiol Technol 27:187–204
Palekar AA, Vasudevan PT, Yan S (2000) Purification of lipase: a review. Biocatal Biotransform 18:177–200
Pandey A, Benjamin S, Soccol CR, Nigam P, Krieger N, Soccol UT (1999) The realm of microbial lipases in biotechnology. Biotechnol Appl Biochem 29:119–131
Patkar SA, Bjorkling F (1994) Lipase inhibitors. In: Woolley P, Petersen SB (eds) Lipases—their structure, biochemistry and application. Cambridge University Press, Cambridge, pp 207–224
Petrounia LP, Arnold FH (2000) Designed evolution of enzymatic properties. Curr Opin Biotechnol 11:325–330
Petschen I, Malo EA, Bosch MP, Guerrero A (1996) Highly enantioselective synthesis of long chain alkyl trifluoromethyl carbinols and β-thiotrio-fluoromethyl carbinols through lipases. Tetrahedron Asymmetry 7:2135–2143
Pratt J, Cooley JD, Purdy CW, Straus DC (2000) Lipase activity from strains of Pasteurella multocida. Curr Microbiol 40:306–309
Pratuamgdejkul J, Dharmsthiti S (2000) Purification and characterization of lipase form psychrophilic Acinetobacter calcoaceticus LP009. Microbiol Res 155:95–100
Queiroz JA, Garcia FAP, Cabral JMS (1995) Hydrophobic interaction chromatography of Chromobacterium viscosum lipase. J Chromatogr A 707:137–142
Queiroz JA, Tomaz CT, Cabral JMS (2001) Hydrophobic interaction chromatography of proteins. J Biotechnol 87:143–159
Rashid N, Shimada Y, Ezaki S, Atomi H, Imanaka T (2001) Low-temperature lipase from psychrotrophic Pseudomonas sp. strain KB700A. Appl Environ Microbiol 67:4064–4069
Rathi P, Bradoo S, Saxena RK, Gupta R (2000) A hyper-thermostable, alkaline lipase from Pseudomonas sp. with the property of thermal activation. Biotechnol Lett 22:495–498
Rathi P, Saxena RK, Gupta R (2001) A novel alkaline lipase from Burkholderia cepacia for detergent formulation. Process Biochem 37:187–192
Rathi P, Goswami VK, Sahai V, Gupta R (2002) Response surface methodology for improving production of hyperthermostable lipase from Burkholderia cepacia. J Appl Microbiol 93:930–936
Reetz MT (2001) Combinatorial and evolution-based methods in the creation of enantioselective catalysts. Angew Chem Int Ed 40:284–310
Reetz MT, Jaeger K-E (1998) Overexpression, immobilization and biotechnological application of Pseudomonas lipases. Chem Phys Lipids 93:3–14
Reetz MT, Jaeger K-E (1999) Superior biocatalysts by directed evolution. Topics Curr Chem 200:31–57
Saxena RK, Sheoran A, Giri B, Davidson S (2003) Purification strategies for microbial lipases. J Microbiol Methods 52:1–18
Schmidt-Dannert C, Sztajer H, Stocklein W, Menge U, Schmid RD (1994) Screening purification and properties of a thermophilic lipase from Bacillus thermocatenulatus. Biochim Biophys Acta 1214:43–53
Schmidt-Dannert C, Rúa ML, Atomi H, Schmid RD (1996) Thermoalkalophilic lipase of Bacillus thermocatenulatus. I. Molecular cloning, nucleotide sequence, purification and some properties. Biochim Biophys Acta 1301:105–114
Schmidt-Dannert C, Luisa Rua M, Schmid RD (1997) Two novel lipases from the thermophile Bacillus thermocatenulatus: Screening, purification, cloning, overexpression and properties. Methods Enzymol 284:194–219
Schuepp C, Kermasha S, Michalski M-C, Morin A (1997) Production, partial purification and characterization of lipases from Pseudomonas fragi CRDA 037. Process Biochem 32:225–232
Schulz T, Plesis J, Schmid RD (2000) Stereoselectivity of Pseudomonas cepacia lipase toward secondary alcohols: a quantitative model. Protein Sci 9:1053–1062
Sharma S, Gupta MN (2001) Alginate as a macroaffinity ligand and an additive for enhanced activity and thermostability of lipases. Biotechnol Appl Biochem 33:161–165
Sharma R, Soni SK, Vohra RM, Gupta LK, Gupta JK (2002a) Purification and characterization of a thermostable alkaline lipase from a new thermophilic Bacillus sp. RSJ-1. Process Biochem 37:1075–1084
Sharma R, Soni SK, Vohra RM, Jolly RS, Gupta LK, Gupta JK (2002b) Production of extracellular alkaline lipase from a Bacillus sp. RSJ1 and its application in ester hydrolysis. Ind J Microbiol 42:49–54
Sharon C, Furugoh S, Yamakido T, Ogawa HI, Kato Y (1998) Purification and characterization of a lipase from Pseudomonas aeruginosa KKA-5 and its role in castor oil hydrolysis. J Ind Microbiol Biotechnol 20:304–307
Shinkai A, Hirano A, Aisaka K (1996) Substitutions of Ser for Asn-163 and Pro for Leu-264 are important for stabilization of lipase from Pseudomonas aeruginosa. J Biochem 120:915–921
Shirazi SH, Rehman SR, Rehman MM (1998) Short communication: production of extracellular lipases by Saccharomyces cerevisiae. World J Microbiol Biotechnol 14:595–597
Sidhu P, Sharma R, Soni SK, Gupta JK (1998a) Effect of cultural conditions on extracellular alkaline lipase production from Bacillus sp. RS-12 and its characterization. Ind J Microbiol 38:9–14
Sidhu P, Sharma R, Soni SK, Gupta JK (1998b) Production of extracellular alkaline lipase by a new thermophilic Bacillus sp. Folia Microbiol 43:51–54
Simons JWFA, Adams H, Cox RC, Dekker N, Gotz F, Slotboom AJ, Verheij HM (1996) The lipase from Staphylococcus aureus: expression in Escherichia coli, large-scale purification and comparison of substrate specificity to Staphylococcus hyicus lipase. Eur J Biochem 242:760–769
Skagerlind P, Jansson M, Hult K (1992) Surfactant interference on lipase catalyzed reactions in microemulsions. J Chem Tech Biotechnol 54:277–282
Snellman EA, Sullivan ER, Colwell RR (2002) Purification and properties of the extracellular lipase, Lip A, of Acinetobacter sp. RAG-1. Eur J Biochem 269:5771–5779
Sugihara A, Tani T, Tominaga Y (1991) Purification and characterization of a novel thermostable lipase from Bacillus sp. J Biochem 109:211–216
Sugiura M, Isobe M, Muroya N, Yamaguchi T (1974) Purification and properties of a Chromobacterium lipase with a high molecular weight. Agric Biol Chem 38:947–952
Sugiura M, Oikawa T, Hirano K, Inukai T (1977) Purification, crystallization and properties of triacylglycerol lipase from Pseudomonas fluorescens. Biochim Biophys Acta 488:353–358
Sunna A, Hunter L, Hutton CA, Bergquist PL (2002) Biochemical characterization of a recombinant thermoalkalophilic lipase and assessment of its substrate enantioselectivity. Enzyme Microb Technol 31:472–476
Surinenaite B, Bendikiene V, Juodka B, Bachmatova I, Marcinkevichiene L (2002) Characterization and physicochemical properties of a lipase from Pseudomonas mendocina 3121-1. Biotechnol Appl Biochem 36:47–55
Swaisgood HE, Bozoglu F (1984) Heat inactivation of the extracellular lipase from Pseudomonas fluorescens MC50. J Agric Food Chem 32:7–10
Taipa MA, Aires-Barros MR, Cabral JMS (1992) Purification of lipases. J Biotechnol 26:111–142
Takagi Y, Teramoto J, Kihara H, Itoh T, Tsukube H (1996) Thiacrown ether as regulator of lipase-catalyzed trans-esterification in organic media—practical optical resolution of allyl alcohols. Tetrahedron Lett 37:4991–4992
Terstappen GC, Geerts AJ, Kula MR (1992) The use of detergent-based aqueous two-phase systems for the isolation of extracellular proteins: purification of a lipase from Pseudomonas cepacia. Biotechnol Appl Biochem 16:228–235
Tobin MB, Gustafsson C, Huisman GW (2000) Directed evolution: the ‘rational’ basis for ‘irrational’ design. Curr Opin Struc Biol 10:421–427
Toyo-Jozo (1988) Production of optically active β-monoalkyl malate and optically active isoserine. Japanese patent JP-J63137687
Van Kampen MD, Rosenstein R, Götz F, Egmond MR (2001) Cloning, purification and characterization of Staphylococcus warneri lipase 2. Biochim Biophys Acta 1544:229–241
Van Oort MG, Deever AMTJ, Dijkman R, Tjeenk ML, Verheij HM, Haas GH de, Wenzig E, Gotz F (1989) Purification and substrate specificity of Staphylococcus hyicus lipase. Biochemistry 28:9278–9285
Vicente MLC, Aires–Barres MR, Cabral JMS (1990) Purification of Chromobacterium viscosum lipases using reverse micelles. Biotechnol Techn 4:137–142
Wakelin NG, Forster CF (1997) An investigation into microbial removal of fats, oils and greases. Bioresour Technol 59:37–43
Wang C-S, Dashti A, Downs D (1999) Bile salt-activated lipase. In: Doolittle MH, Reue K (eds) Lipase and phospholipase protocols. (Methods in molecular biology, vol 109) Humana Press, Totowa, N.J., pp 71–79
Wang Y, Srivastava KC, Shen G-J, Wang HY (1995) Thermostable alkaline lipase from a newly isolated thermophilic Bacillus, strain A30-1 (ATCC 53841). J Ferment Bioeng 79:433–438
Yamada Y, Kuboi R, Komasawa I (1993) Increased activity of Chromobacterium viscosum lipase in aerosol OT reverse micelles in the presence of nonionic surfactants. Biotechnol Prog 9:468–472
Yamamoto K, Fujiwara N (1988) Purification and some properties of a castor-oil-hydrolysing lipase from Pseudomonas sp. Agric Biol Chem 52:3015–3021
Yamamoto K, Fujiwara N (1995) The hydrolysis of castor oil using a lipase from Pseudomonas sp. FB-24: positional and substrate specificity of the enzyme and optimum reaction conditions. Biosci Biotechnol Biochem 59:1262–1266
Yeo SH, Nihira T, Yamada Y (1998) Screening and identification of a novel lipase form Burkholderia sp. YY62 which hydrolyzes t-butyl esters effectively. J Gen Appl Microbiol 44:147–152
Zhao H, Chockalingam K, Chen Z (2002) Directed evolution of enzymes and pathways for industrial biocatalysis. Curr Opin Biotechnol 13:104–110
Acknowledgement
The authors thank the Department of Biotechnology, New Delhi (Government of India) for financial assistance through a project on lipase from Burkholderia sp. (Sanction No. BT/PR2742/PID/04/127/2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, R., Gupta, N. & Rathi, P. Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol 64, 763–781 (2004). https://doi.org/10.1007/s00253-004-1568-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1568-8