Abstract
Purpose
The purpose of this study is to determine the rate of QTcP and associated risk factors in patients treated with voriconazole.
Methods
We conducted a retrospective chart review of all patients treated with voriconazole in a large tertiary center between 2009 and 2015, using paired comparison of QTc intervals on and off voriconazole treatment, adjusted for comorbidities, electrolyte abnormalities, and concurrent medications.
Results
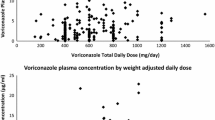
Fifty-four patients were included, of whom 53 were diagnosed with oncologic/hemato-oncologic disease. Mean QTc during voriconazole therapy (448.0 ± 52.9 msec) was significantly longer compared to QTc off voriconazole (421.8 ± 42.2 msec; p = 0.002). QTcP ≥30 msec and ≥60 msec was demonstrated in 43% (23 patients) and 28% (15 patients), respectively. Multivariate analysis showed that QTcP was significantly associated with baseline QTc ≥ 450 msec (upper QTc quartile) (p < 0.01) and low serum potassium levels (p < 0.01). Contrarily, no significant association was found between mean voriconazole daily and cumulative dose and QTcP.
Conclusion
Our findings indicate that hemato-oncologic patients treated with voriconazole are at increased risk for QTcP, especially in the presence of baseline QTc ≥ 450 msec and low serum potassium levels.
Similar content being viewed by others
References
Walsh TJ, Anaissie EJ, Denning DW et al (2008) Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 46:327–360
Boyd AE, Modi S, Howard SJ, Moore CB, Keevil BG, Denning DW (2004) Adverse reactions to voriconazole. Clin Infect Dis 39:1241–1244
Willis ZI, Boyd AS, Di Pentima MC (2015) Phototoxicity, pseudoporphyria and photo-onycholysis due to voriconazole in a pediatric patient with leukemia and invasive aspergillosis. J pediatric Infect Dis Soc 4:e22–e24
Alkan Y, Haefeli WE, Burhenne J, Stein J, Yaniv I, Shalit I (2004) Voriconazole-induced QT interval prolongation and ventricular tachycardia: a non-concentration-dependent adverse effect. Clin Infect Dis 39:e49–e52
Aypar E, Kendirli T, Tutar E et al (2011) Voriconazole-induced QT interval prolongation and torsades de pointes. Pediatr Int 53:761–763
Philips JA, Marty FM, Stone RM, Koplan BA, Katz JT, Baden LR (2007) Torsades de pointes associated with voriconazole use. Transpl Infect Dis 9:33–36
Elbey MA, Cil H, Onturk E, Islamoglu Y (2012) QTc prolongation and torsade de pointes ventricular tachycardia in a small dose voriconazole therapy. Eur Rev Med Pharmacol Sci 16:100–102
Brown JD, Lim LL, Koning S (2014) Voriconazole associated torsades de pointes in two adult patients with haematological malignancies. Med Mycol Case Rep 13:23–25
Zeuli JD, Wilson JW, Estes LL (2013) Effect of combined fluoroquinolone and azole use on QT prolongation in hematology patients. Antimicrob Agents Chemother 57:1121–1127
Behr ER, Roden D (2013) Drug-induced arrhythmia: pharmacogenomic prescribing? Eur Heart J 34:89–95
Choo WK, Turpie D, Milne K et al (2014) Prescribers’ practice of assessing arrhythmia risk with QT-prolonging medications. Cardiovasc Ther 32:209–213
Viskin S, Havakuk O, Schwaber MJ (2015) Pro-arrhythmic effects of noncardiac medications: lessons from macrolide antibiotics. J Am Coll Cardiol 66:2185–2188
Polak S, Wiśniowska B, Brandys J (2009) Collation, assessment and analysis of literature in vitro data on hERG receptor blocking potency for subsequent modeling of drugs’ cardiotoxic properties. J Appl Toxicol 29:183–206
ICH expert working group (2015) The clinical evaluation of QT/QTC interval prolongation and proarrhythmic potential for nonantiarrhythmic drugs E14. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Switzerland, Geneva http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf. Accessed 20 August 2016
FDA antiviral drugs advisory committee. Briefing document for voriconazole (oral and intravenous formulations).
Han S, Zhang Y, Chen Q et al (2011) Fluconazole inhibits hERG K(+) channel by direct block and disruption of protein trafficking. Eur J Pharmacol 650:138–144
Colatsky T, Fermini B, Gintant G et al (2016) The comprehensive in vitro proarrhythmia assay (CiPA) initiative—update on progress. J Pharmacol Toxicol Methods. doi:10.1016/j.vascn.2016.06.002
Woosley RL, Romero KA. www.Crediblemeds.org , QTdrugs List, Access Date: 07 April 2016, AZCERT, Inc. 1822 Innovation Park Dr., Oro Valley, AZ 85755
Pascual A, Nieth V, Calandra T et al (2007) Variability of voriconazole plasma levels measured by new high-performance liquid chromatography and bioassay methods. Antimicrob Agents Chemother 51:137–143
Yap YG, Camm AK (2003) Drug induced QT prolongation and torsades de pointes. Heart 89:1363–1372
Loung ML, Al-Dabbagh M, Groll AH et al (2016) Utility of voriconazole therapeutic drug monitoring: a meta-analysis. J Antimicrob Chemother 71:1786–1799
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gueta, I., Loebstein, R., Markovits, N. et al. Voriconazole-induced QT prolongation among hemato-oncologic patients: clinical characteristics and risk factors. Eur J Clin Pharmacol 73, 1181–1185 (2017). https://doi.org/10.1007/s00228-017-2284-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2284-5