Abstract
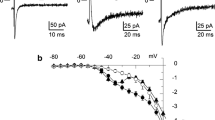
Adrenergic activation of L-type Ca2+ and various K+ currents is a crucial mechanism of cardiac adaptation; however, it may carry a substantial proarrhythmic risk as well. The aim of the present work was to study the timing of activation of Ca2+ and K+ currents in isolated canine ventricular cells in response to exposure to isoproterenol (ISO). Whole cell configuration of the patch-clamp technique in either conventional voltage clamp or action potential voltage clamp modes were used to monitor I Ca, I Ks, and I Kr, while action potentials were recorded using sharp microelectrodes. ISO (10 nM) elevated the plateau potential and shortened action potential duration (APD) in subepicardial and mid-myocardial cells, which effects were associated with multifold enhancement of I Ca and I Ks and moderate stimulation of I Kr. The ISO-induced plateau shift and I Ca increase developed faster than the shortening of APD and stimulation of I Ks and I Kr. Blockade of β1-adrenoceptors (using 300 nM CGP-20712A) converted the ISO-induced shortening of APD to lengthening, decreased its latency, and reduced the plateau shift. In contrast, blockade of β2-adrenoceptors (by 50 nM ICI 118,551) augmented the APD-shortening effect and increased the latency of plateau shift without altering its magnitude. All effects of ISO were prevented by simultaneous blockade of both receptor types. Inhibition of phosphodiesterases decreased the differences observed in the turn on of the ISO-induced plateau shift and APD shortening. ISO-induced activation of I Ca is turned on faster than the stimulation of I Ks and I Kr in canine ventricular cells due to the involvement of different adrenergic pathways and compartmentalization.






Similar content being viewed by others
References
Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ (2006) Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for β2-adrenergic regulation. PNAS 103:7500–7505. doi:10.1073/pnas.0503465103
Bányász T, Fülöp L, Magyar J, Szentandrássy N, Varró A, Nánási PP (2003) Endocardial versus epicardial differences in L-type calcium current in canine ventricular myocytes studied by action potential voltage clamp. Cardiovasc Res 58:66–75. doi:10.1016/S0008-6363(02)00853-2
Bányász T, Magyar J, Szentandrássy N, Horváth B, Birinyi P, Szentmiklósi J, Nánási PP (2007) Action potential clamp fingerprints of K+ currents in canine cardiomyocytes: their role in ventricular repolarization. Acta Physiol Scand 190:189–198. doi:10.1111/j.1748-1716.2007.01674.x
Best JM, Kamp TJ (2012) Different subcellular populations of L-type Ca2+ channels exhibit unique regulation and functional roles in cardiomyocytes. J Mol Cell Cardiol 52:376–387. doi:10.1016/j.yjmcc.2011.08.014
Chen-Izu Y, Xiao R-P, Izu LT, Cheng H, Kuschel M, Spurgeon H, Lakatta EG (2000) Gi-dependent localization of β2–adrenergic receptor signaling to L-type Ca2+ channels. Biophys J 79:2547–2556. doi:10.1016/S0006-3495(00)76495-2
Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, Horne MC, Hoshi T, Hell JW (2001) A β2-adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science 293:98–101. doi:10.1126/science.293.5527.98
Doerr T, Denger R, Doerr A, Trautwein W (1990) Ionic currents contributing to the action potential in single ventricular myocytes of the guinea pig studied with action potential clamp. Pflugers Arch 416:230–237. doi:10.1007/BF00392058
Fischmeister R, DeFelice LJ, Ayer RK, Levi R, DeHaan RL (1984) Channel currents during spontaneous action potentials in embryonic chick heart sells. The action potential patch clamp. Biophys J 46:267–271. doi:10.1016/S0006-3495(84)84020-5
Frace M, Méry P-F, Fischmeister R, Hartzell HC (1993) Rate-limiting steps in the ß-adrenergic stimulation of cardiac calcium current. J Gen Physiol 101:337–353. doi:10.1085/jgp.101.3.337
Harada K, Iijima T (1994) Differential modulation by adenylate cyclase of Ca2+ and delayed K+ current in ventricular myocytes. Am J Physiol 266:H1551–H1557
Harmati G, Bányász T, Bárándi L, Szentandrássy N, Horváth B, Szabó G, Szentmiklósi JA, Szénási G, Nánási PP, Magyar J (2011) Effects of ß-adrenergic stimulation on delayed rectifier K+ currents in canine ventricular cardiomyocytes. Br J Pharmacol 162:890–896. doi:10.2174/0929867043456214
Harvey RD, Clark CD, Hume JR (1990) Cl− current in mammalian cardiac myocytes: novel mechanism for autonomic regulation of action potential duration and resting membrane potential. J Gen Physiol 95:1077–1102. doi:10.1085/jgp.95.6.1077
Heath BM, Terrar DA (2000) Protein kinase C enhances the rapidly activating delayed rectifier potassium current, IKr, through a reduction in C-type inactivation in guinea-pig ventricular myocytes. J Physiol Lond 522:391–402. doi:10.1111/j.1469-7793.2000.t01-2-0039
Heijman J, Volders PGA, Westra RL, Rudy Y (2011) Local control of β-adrenergic stimulation: effects on ventricular myocyte electrophysiology and Ca2+-transient. J Mol Cell Cardiol 50:863–871. doi:10.1016/j.yjmcc.2011.02.007
January CT, Riddle MJ (1990) Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res 64:977–990. doi:10.1161/01.RES.64.5.977
Jost N, Acsai K, Horváth B, Bányász T, Bitay M, Bogáts G, Nánási PP (2009) Contribution of IKr and IK1 to ventricular repolarization in canine and human myocytes. Is there any influence of action potential duration? Basic Res Cardiol 104:33–41. doi:10.1007/s00395-008-0730-3
Kaumann AJ, Lemoine H (1987) β2-adrenoceptor-mediated positive inotropic effect of adrenaline in human ventricular myocardium. Quantitative discrepancies with binding and adenylate cyclase stimulation. Naunyn-Schmiedeberg’s Arch Pharmacol 335:403–411. doi:10.1007/BF00165555
Kuschel M, Zhou YY, Cheng H, Zhang SJ, Chen Y, Lakatta EG (1999a) Gi protein-mediated functional compartmentalization of cardiac β2–adrenergic signaling. J Biol Chem 274:22048–22052. doi:10.1074/jbc.274.31.22048
Kuschel M, Zhou YY, Spurgeon HA, Bartel S, Karczewski P, Zhang SJ, Krause EG, Lakatta EG, Xiao RP (1999b) β2-adrenergic cAMP signaling is uncoupled from phosphorylation of cytoplasmic proteins in canine heart. Circulation 99:2458–2465. doi:10.1161/01.CIR.99.18.2458
Lemoine H, Ehle B, Kaumann AJ (1985) Direct labeling of β2–adrenoceptors. Comparison of binding potency of 3H-ICI 118,551 and blocking potency of ICI 118,551. Naunyn Schmiedeberg’s Arch Pharmacol 331:40–51. doi:10.1007/BF00498850
Liu X-G, Choi B-R, Ziv O, Li W, de Lange E, Qu Z, Koren G (2012) Differential conditions for early after-depolarizations and triggered activity in cardiomyocytes derived from transgenic LQT1 and LQT2 rabbits. J Physiol Lond 590(5):1171–1180. doi:10.1113/jphysiol.2011.218164
McDonald TF, Pelzer S, Trautwein W, Pelzer DJ (1994) Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev 74:365–507
Méry P-F, Frace M, Hartzell HC, Fischmeister R (1993) A comparative analysis of the time course of cardiac Ca2+ current response to rapid applications of ß-adrenergic and dihydropyridine agonists. Naunyn Schmiedeberg’s Arch Pharmacol 348:197–206. doi:10.1007/BF00164799
Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J (2010) β2-adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327:1653–1657. doi:10.1126/science.1185988
Post SR, Hammond HK, Insel PA (1999) β-adrenergic receptors and receptor signaling in heart failure. Annu Rev Pharmacol Toxicol 39:343–360. doi:10.1146/annurev.pharmtox.39.1.343
Rocchetti M, Freli V, Perego V, Altomare C, Mostacciuolo G, Zaza A (2006) Rate dependency of beta-adrenergic modulation of repolarizing currents in the guinea-pig ventricle. J Physiol (Lond) 574:183–193. doi:10.1113/jphysiol.2006.105015
Simkó J, Szentandrássy N, Harmati G, Bárándi L, Horváth B, Magyar J, Bányász T, Lőrincz I, Nánási PP (2010) Effects of ropinirole on action potential characteristics and the underlying ion currents in canine ventricular myocytes. Naunyn Schmiedeberg’s Arch Pharmacol 382:213–220. doi:10.1007/s00210-010-0538-1
Skeberdis VA, Jurevicius J, Fischmeister R (1997) β2-adrenergic activation of L-type Ca2+ current in cardiac myocytes. J Pharmacol Exp Ther 283:452–461
Steinberg SF, Brunton LL (2001) Compartmentalization of G protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol 41:751–773. doi:10.1146/annurev.pharmtox.41.1.751
Stengl M, Volders PGA, Thomsen MB, Spatjens RLHMG, Sipido KR, Vos M (2003) Accumulation of slowly activating delayed rectifier potassium current (IKs) in canine ventricular myocytes. J Physiol Lond 551:777–786. doi:10.1113/jphysiol.2003.044040
Szabó G, Szentandrássy N, Bíró T, Tóth IB, Czifra G, Magyar J, Bányász T, Varró A, Kovács L, Nánási PP (2005) Asymmetrical distribution of ion channels in canine and human left ventricular wall: epicardium versus midmyocardium. Pflugers Arch 450:307–316. doi:10.1007/s00424-005-1445-z
Szabó L, Szentandrássy N, Kistamás K, Hegyi B, Ruzsnavszky F, Váczi K, Horváth B, Magyar J, Bányász T, Pál B, Nánási PP (2013) Effects of tacrolimus on action potential configuration and transmembrane ion currents in canine ventricular cells. Naunyn Schmiedeberg’s Arch Pharmacol 386:239–246. doi:10.1007/s00210-012-0823-2
Szentadrássy N, Bányász T, Bíró T, Szabó G, Tóth B, Magyar J, Lázár J, Varró A, Kovács L, Nánási PP (2005) Apico-basal inhomogeneity in distribution of ion channels in canine and human ventricular myocardium. Cardiovasc Res 65:851–860. doi:10.1016/j.cardiores.2004.11.022
Szentandrássy N, Farkas V, Bárándi L, Hegyi B, Ruzsnavszky F, Horváth B, Bányász T, Magyar J, Márton I, Nánási PP (2012) Role of action potential configuration and the contribution of Ca2+ and K+ currents to isoprenaline-induced changes in canine ventricular cells. Br J Pharmacol 167:599–611. doi:10.1111/j.1476-5381.2012.02015.x
Vandecasteele G, Rochais F, Abi-Gerges A, Fischmeister R (2006) Functional localization of cAMP signalling in cardiac myocytes. Biochem Soc Trans 34:484–488. doi:10.1042/BST0340484
Van der Heyden MA, Wijnhoven TJ, Opthof T (2005) Molecular aspects of adrenergic modulation of cardiac L-type Ca2+ channels. Cardiovasc Res 65:28–39. doi:10.1016/j.cardiores.2004.09.028
Varro A, Balati B, Iost N, Takacs J, Virag L, Lathrop DA, Cs L, Tálosi L, Papp JGY (2000) The role of the delayed rectifier component IKs in dog ventricular muscle and Purkinje fibre repolarization. J Physiol Lond 523:67–81. doi:10.1111/j.1469-7793.2000.00067.x
Volders PGA, Stengl M, van Opstal JM, Gerlach U, Spatjens RLHMG, Beekman JDM, Sipido KR, Vos MA (2003) Probing the contribution of IKs to canine ventricular repolarization: key role for β-adrenergic receptor stimulation. Circulation 107:2753–2760. doi:10.1161/01.CIR.0000068344.54010.B3
Walsh KB, Begenisich T, Kass RS (1988) β-adrenergic modulation in the heart. Independent regulation of K and Ca channels. Pflugers Arch 411:232–234
Xiao RP (2001) β-adrenergic signaling in the heart: dual coupling of the β-adrenergic receptor to Gs and Gi proteins. Sci STKE 104:re15. doi:10.1126/stke.2001.104.re15
Xiao RP, Cheng H, Zhou YY, Kuschel M, Lakatta EG (1999) Recent advances in cardiac β2-adrenergic signal transduction. Circ Res 85:1092–1100. doi:10.1161/01.RES.85.11.1092
Xiao RP, Hohl C, Altschuld R, Jones L, Livingston B, Ziman B, Tantini B, Lakatta EG (1994) β2-adrenergic receptor-stimulated increase in cAMP in rat heart cells is not coupled to changes in Ca2+ dynamics, contractility, or phospholamban phosphorylation. J Biol Chem 269:19151–19156
Xiao RP, Ji X, Lakatta EG (1995) Functional coupling of the β2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol Pharmacol 47:322–329. doi:10.1161/01.RES.84.1.43
Xiao RP, Lakatta EG (1993) β1-adrenoceptor stimulation and β2-adrenoceptor stimulation differ in their effects on contraction, cytosolic Ca2+, and Ca2+ current in single rat ventricular cells. Circ Res 73:286–300. doi:10.1161/01.RES.73.2.286
Xie Y, Grandi E, Puglisi JL, Sato D, Bers DM (2013) β-adrenergic stimulation activates early afterdepolarizations transiently via kinetic mismatch of PKA targets. J Mol Cell Cardiol 58:153–161
Zhu Y, Ai X, Oster RA, Bers DM, Pogwizd SM (2013) Sex differences in repolarization and slow delayed rectifier potassium current and their regulation by sympathetic stimulation in rabbits. Pflugers Arch 465:805–818. doi:10.1007/s00424-012-1193-9
Acknowledgments
Financial support was provided by grants from the Hungarian Scientific Research Fund (OTKA-K100151, OTKA-K101196, OTKA-PD101171, and OTKA-NK104331). Further support was obtained from the Hungarian Government (TAMOP-4.2.2.A-11/1/KONV-2012-0045 and TAMOP-4.2.2/B-10/1-2010-0024 research projects). The authors thank Miss Éva Sági for her excellent technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruzsnavszky, F., Hegyi, B., Kistamás, K. et al. Asynchronous activation of calcium and potassium currents by isoproterenol in canine ventricular myocytes. Naunyn-Schmiedeberg's Arch Pharmacol 387, 457–467 (2014). https://doi.org/10.1007/s00210-014-0964-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-014-0964-6