Abstract
Summary
Triple A syndrome (alacrima, achalasia, adrenal failure, progressive neurodegenerative disease) is caused by mutations in the AAAS gene which encodes the protein alacrima achalasia adrenal insufficiency neurologic disorder (ALADIN). Our investigation suggests that low bone mineral density (BMD) for age/osteoporosis could be a common but overlooked symptom of unexplained etiology in this rare multisystemic disease.
Introduction
The purpose of this study is to evaluate incidence and etiology of BMD for age/osteoporosis, a possibly overlooked symptom in triple A syndrome.
Methods
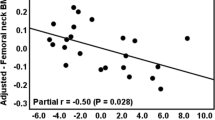
Dual-energy X-ray absorptiometry (DXA) of the femoral neck, total hip, lumbar spine, and radius, bone turnover markers, minerals, total alkaline phosphatase (ALP), 25-hydroxy vitamin D (25-OHD), 1,25-dihydroxy vitamin D (1,25-OH2D), intact parathyroid hormone (PTH), and adrenal androgens (dehydroepiandrosterone sulfate (DHEAS) and androstenedione) were measured in five male and four female patients.
Results
At time of diagnosis, low BMD for age was suspected on X-ray in seven of nine patients aged 2–11 years (not performed in two patients); normal levels of minerals and ALP were found in nine patients and low levels of adrenal androgens in eight patients (not measured in one patient). Reevaluation 5–35 years after introduction of 12 mg/m2/day hydrocortisone showed low BMD for age in two children, osteopenia in one, and osteoporosis in six adults. Normal levels of minerals, ALP, PTH, 1,25-OH2D, procollagen type 1, crosslaps, and osteocalcin were found in all patients. Low levels of adrenal androgens were found in all and 25OHD deficiency in six patients. Body mass index was <25 % for age and sex in eight of nine patients.
Conclusion
Low BMD for age/osteoporosis in our patients probably is not a result of glucocorticoid therapy but could be the consequence of low level of adrenal androgens, neurological impairment causing physical inactivity, inadequate sun exposure, and protein malnutrition secondary to achalasia. Considering ubiquitous ALADIN expression, low BMD/osteoporosis may be a primary phenotypic feature of the disease. Besides optimizing glucocorticoid dose, physical activity, adequate sun exposure, appropriate nutrition, and vitamin D supplementation, therapy with DHEA should be considered.
Similar content being viewed by others
References
Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ (2002) Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol 158:915–927
Dreger M, Bengtsson L, Schoneberg T, Otto H, Hucho F (2001) Nuclear envelope proteomics: novel integral membrane proteins of the inner nuclear membrane. Proc Natl Acad Sci U S A 98:11943–11948
Kind B, Koehler K, Lorenz M, Huebner A (2009) The nuclear pore complex protein ALADIN is anchored via NDC1 but not via POM121 and GP210 in the nuclear envelope. Biochem Biophys Res Commun 390:205–210
Krumbholz M, Koehler K, Huebner A (2006) Cellular localization of 17 natural mutant variants of ALADIN protein in triple A syndrome—shedding light on an unexpected splice mutation. Biochem Cell Biol 84:243–249
Tullio-Pelet A, Salomon R, Hadj-Rabia S, Mugnier C, De Laet MH, Chaouachi B, Bakiri F, Brottier P, Cattolico L, Penet C, Begeot M, Naville D, Nicolino M, Chaussain JL, Weissenbach J, Munnich A, Lyonnet S (2000) Mutant WD-repeat protein in triple-A syndrome. Nat Genet 26:332–335
Handschug K, Sperling S, Yoon SJ, Hennig S, Clark AJ, Huebner A (2001) Triple A syndrome is caused by mutations in AAAS, a new WD-repeat protein gene. Hum Mol Genet 10:283–290
Huebner A, Kaindl AM, Knobeloch KP, Petzold H, Mann P, Koehler K (2004) The triple A syndrome is due to mutations in ALADIN, a novel member of the nuclear pore complex. Endocr Res 30:891–899
Brooks BP, Kleta R, Stuart C, Tuchman M, Jeong A, Stergiopoulos SG, Bei T, Bjornson B, Russell L, Chanoine JP, Tsagarakis S, Kalsner L, Stratakis C (2005) Genotypic heterogeneity and clinical phenotype in triple A syndrome: a review of the NIH experience 2000–2005. Clin Genet 68:215–221
Yassaee VR, Soltani Z, Ardakani BM (2011) Mutation spectra of the AAAS gene in Iranian families with Allgrove syndrome. Arch Med Res 42:163–168
Ikeda M, Hirano M, Shinoda K, Katsumata N, Furutama D, Nakamura K, Ikeda S, Tanaka T, Hanafusa T, Kitajima H, Kohno H, Nakagawa M, Nakamura Y, Ueno S (2013) Triple A syndrome in Japan. Muscle Nerve 48:381–386
Dumić M, Radica A, Sabol Z, Plavsić V, Brkljacić L, Sarnavka V, Vuković J (1991) Adrenocorticotropic hormone insensitivity associated with autonomic nervous system disorders. Eur J Pediatr 150:696–699
Persic M, Prpić I, Huebner A, Severinski S (2001) Achalasia, alacrima, adrenal insufficiency, and autonomic dysfunction: double A, triple A, or quaternary A syndrome? J Pediatr Gastroenterol Nutr 33:503–504
Dumić M, Barišić N, Rojnić-Putarek N, Kušec V, Stanimirović A, Koehler K, Huebner A (2011) Two siblings with triple A syndrome and novel mutation presenting as hereditary polyneuropathy. Eur J Pediatr 170:393–396
Dumic M, Barišic N, Kusec V, Stingl K, Skegro M, Stanimirovic A, Koehler K, Huebner A (2012) Long-term clinical follow-up and molecular genetic findings in eight patients with triple A syndrome. Eur J Pediatr 171:1453–1459
Bachrach LK, Sills IN (2011) Clinical report—bone densitometry in children and adolescents. Pediatrics 127:189–194
WHO (2004) Scientific group on the assessment of osteoporosis at primary healthcare level. Summary Meeting Report Brussels, Belgium, pp 5–7
Ozgen AG, Ercan E, Ozütemiz O, Hamulu F, Bayraktar F, Yilmaz C (1999) The 4A syndrome association with osteoporosis. Endocr J 46:227–230
Dusek T, Korsic M, Koehler K, Perkovic Z, Huebner A, Korsic M (2006) A novel AAAS gene mutation (p.R194X) in a patient with triple A syndrome. Horm Res 65:171–176
Bizzarri C, Benevento D, Terzi C, Huebner A, Cappa M (2013) Triple A (Allgrove) syndrome: an unusual association with syringomyelia. Ital J Pediatr 39:39–43
Palka C, Giuliani R, Brancati F, Mohn A, Di Muzio A, Calabrese O, Huebner A, De Grandis D, Chiarelli F, Ferlini A, Stuppia L (2010) Two Italian patients with novel AAAS gene mutation expand allelic and phenotypic spectrum of triple A (Allgrove) syndrome. Clin Genet 77:298–301
Siebel MJ, Cooper MS, Zhou H (2013) Glucocorticoid-induced osteoporosis: mechanism, management and future perspectives. Lancet Diabetes Endocrinol 58:617–620
Jódar E, Valdepeñas MP, Martinez G, Jara A, Hawkins F (2003) Long-term follow-up of bone mineral density in Addison's disease. Clin Endocrinol 58:617–620
Koetz KR, Ventz M, Diederich S, Quinkler M (2012) Bone mineral density is not significantly reduced in adult patients on low-dose glucocorticoid replacement therapy. J Clin Endocrinol Metab 97:85–92
Clarke BL, Khosia S (2009) Androgens and bone. Steroids 74:296–305
De Oliviera DH, Fighera TM, Bianchet LC, Kulak CA, Kulak J (2012) Androgens and bone. Minerva Endocrinol 37:305–301
Cho AR, Yang KJ, Bae Y, Bahk YY, Kim E, Lee H, Kim JK, Park W, Rhim H, Choi SY, Imanaka T, Moon S, Yoon J, Yoon SK (2009) Tissue-specific expression and subcellular localization of ALADIN, the absence of which causes human triple A syndrome. Exp Mol Med 41:381–386
Gurnell EM, Hunt PJ, Curran SE, Conway CL, Pullenaygum EM, Huppert FA, Compston JE, Herbert J, Chatterjee VK (2008) J Clin Endocrinol Metab 93:400–409
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dumic, M., Putarek, N.R., Kusec, V. et al. Low bone mineral density for age/osteoporosis in triple A syndrome—an overlooked symptom of unexplained etiology. Osteoporos Int 27, 521–526 (2016). https://doi.org/10.1007/s00198-015-3265-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-015-3265-0