Abstract
Key message
Identification and molecular analysis of four tribenuron-methyl resistant mutants in Brassica napus , which would be very useful in hybrid production using a Chemically induced male sterility system.
Abstract
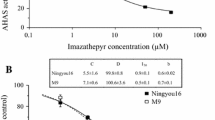
Chemically induced male sterility (CIMS) systems dependent on chemical hybridization agents (CHAs) like tribenuron-methyl (TBM) represent an important approach for practical utilization of heterosis in rapeseed. However, when spraying the female parents with TBM to induce male sterility the male parents must be protected with a shield to avoid injury to the stamens, which would otherwise complicate the seed production protocol and increase the cost of hybrid seed production. Here we report the first proposed application of a herbicide-resistant cultivar in hybrid production, using a CIMS system based on identifying four TBM-resistant mutants in Brassica napus. Genetic analysis indicated that the TBM resistance was controlled by a single dominant nuclear gene. An in vitro enzyme activity assay for acetohydroxyacid synthase (AHAS) suggested that the herbicide resistance is caused by a gain-of-function mutation in a copy of AHAS genes. Comparative sequencing of the mutants and wild type BnaA.AHAS.a coding sequences identified a C-to-T transition at either position 535 or 536 from the translation start site, which resulted in a substitution of proline with serine or leucine at position 197 according to the Arabidopsis thaliana protein sequence. An allele-specific dCAPS marker developed from the C536T variation co-segregated with the herbicide resistance. Transgenic A. thaliana plants expressing BnaA.ahas3.a conferred herbicide resistance, which confirmed that the P197 substitution in BnaA.AHAS.a was responsible for the herbicide resistance. Moreover, the TBM-resistant lines maintain normal male fertility under TBM treatment and can be of practical value in hybrid seed production using CIMS.






Similar content being viewed by others
References
Cheng F, Liu SY, Wu J, Fang L, Sun SL, Liu B, Li PX, Hua W, Wang XW (2011) BRAD, the genetics and genomics database for Brassica plants. BMC Plant Biol 11:136
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Cui HL, Zhang CX, Wei SH, Zhang HJ, Li XJ, Zhang YQ, Wang GQ (2011) Acetolactate synthase gene proline (197) mutations confer tribenuron-methyl resistance in flixweed (Descurainia sophia) populations from China. Weed Sci 59:376–379
Duggleby RG, Pang SS (2000) Acetohydroxyacid synthase. J Biochem Mol Biol 33:1–36
Duggleby RG, McCourt JA, Guddat LW (2008) Structure and mechanism of inhibition of plant acetohydroxyacid synthase. Plant Physiol Biochem 46:309–324
Fan ZX, Lei WX, Hong DF, He JP, Wan LL, Xu ZH, Liu PW, Yang GS (2007) Development and primary genetic analysis of a fertility temperature-sensitive polima cytoplasmic male sterility restorer in Brassica napus. Plant Breed 126:297–301
Ghio C, Ramos ML, Altieri E, Bulos M, Sala CA (2013) Molecular characterization of Als1, an acetohydroxyacid synthase mutation conferring resistance to sulfonylurea herbicides in soybean. Theor Appl Genet 126:2957–2968
Guan CY, Stringam GR (1998) The effect of ZMA on inducing male sterility on spring canola. Crucif Newsl 20:55–56
Hattori J, Brown D, Mourad G, Labbé H, Ouellet T, Sunohara G, Rutledge R, King J, Miki B (1995) An acetohydroxy acid synthase mutant reveals a single site involved in multiple herbicide resistance. Mol Gen Genet 246:419–425
Haughn GW, Smith J, Mazur B, Somerville C (1988) Transformation with a mutant Arabidopsis acetolactate synthase gene renders tobacco resistant to sulfonylurea herbicides. Mol Gen Genet 211:266–271
Hu ML, Pu HM, Gao JQ, Long WH, Qi CK, Zhang JF, Chen S (2012) Inheritance and gene cloning of an ALS inhabiting herbicide-resistant mutant line M9 in Brassica napus. Scientia Agricultura Sinica 45:4326–4334 (in Chinese)
Huang Z, Chen YF, Yi B, Xiao L, Ma CZ, Tu JX, Fu TD (2007) Fine mapping of the recessive genic male sterility gene (Bnms3) in Brassica napus L. Theor Appl Genet 115:113–118
Li Z, Liu H, Luo P (1995) Production and cytogenetics of intergeneric hybrids between Brassica napus and Orychophragmus violaceus. Theor Appl Genet 91:131–136
Liu Z, Guan C, Zhao F, Chen S (2005) Inheritance and mapping of a restorer gene for the rapeseed cytoplasmic male sterile line 681A. Plant Breed 124:5–8
Liu S, Liu Y, Yang X, Tong C, Edwards D, Parkin IAP, Zhao M, Ma J, Yu J, Huang S, Wang X, Wang J, Lu K, Fang Z, Bancroft I, Yang TJ, Hu Q, Wang X, Yue Z, Li H, Yang L, Wu J, Zhou Q, Wang W, King GJ, Pires JC, Lu C, Wu Z, Sampath P, Wang Z, Guo H, Pan S, Yang L, Min J, Zhang D, Jin D, Li W, Belcram H, Tu J, Guan M, Qi C, Du D, Li J, Jiang L, Batley J, Sharpe AG, Park B-S, Ruperao P, Cheng F, Waminal NE, Huang Y, Dong C, Wang L, Li J, Hu Z, Zhuang M, Huang Y, Huang J, Shi J, Mei D, Liu J, Lee T-H, Wang J, Jin H, Li Z, Li X, Zhang J, Xiao L, Zhou Y, Liu Z, Liu X, Qin R, Tang X, Liu W, Wang Y, Zhang Y, Lee J, Kim HH, Denoeud F, Xu X, Liang X, Hua W, Wang X, Wang J, Chalhoub B, Paterson AH (2014) The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat Commun 5:3930
McCourt JA, Duggleby RG (2006) Acetohydroxyacid synthase and its role in the biosynthetic pathway for branched-chain amino acids. Amino Acids 31:173–210
McCourt JA, Pang SS, King-Scott J, Guddat LW, Duggleby RG (2006) Herbicide-binding sites revealed in the structure of plant acetohydroxyacid synthase. Proc Natl Acad Sci USA 103:569–573
McVetty PBE (1997) Cytoplasmic male sterility. In: Shivanna KR, Sawhney VK (eds) Pollen Biotechnology for Crop Production and Improvement. Cambridge University Press, United Kingdom, pp 155–182
Neff MM, Turk E, Kalishman M (2002) Web-based primer design for single nucleotide polymorphism analysis. Trends Genet 18:613–615
Ouellet T, Rutledge RG, Miki BL (1992) Members of the acetohydroxyacid synthase multigene family of Brassica napus have divergent patterns of expression. Plant J 2:321–330
Powles SB, Yu Q (2010) Evolution in action: plants resistant to herbicides. Annu Rev Plant Biol 61:317–347
Rutledge RG, Quellet T, Hattori J, Miki BL (1991) Molecular characterization and genetic origin of the Brassica napus acetohydroxyacid synthase multigene family. Mol Gen Genet 229:31–40
Sala CA, Bulos M (2012) Inheritance and molecular characterization of broad range tolerance to herbicides targeting acetohydroxyacid synthase in sunflower. Theor Appl Genet 124:355–364
Schägger H, Von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Ana Biochem 166:368–379
Shull GH (1908) The composition of a field of maize. J Hered 4:296–301
Sibony M, Michel A, Haas HU, Rubin B, Hurle K (2001) Sulfometuron-resistant Amaranthus retroflexus: cross-resistance and molecular basis for resistance to acetolactate synthase-inhibiting herbicides. Weed Res 41:509–522
Singh BK, Stidham MA, Shaner DL (1988) Assay of acetohydroxyacid synthase. Ana. Biochem 171:173–179
Swanson EB, Coumans MP, Brown GL, Patel JD, Beversdorf WD (1988) The characterization of herbicide tolerant plants in Brassica napus L. after in vitro selection of microspores and protoplasts. Plant Cell Rep 7:83–87
Swanson EB, Herrgesell MJ, Arnoldo M, Sippell DW, Wong RSC (1989) Microspore mutagenesis and selection: canola plants with field tolerance to the imidazolinones. Theor Appl Genet 78:525–530
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tan SY, Evans RR, Dahmer ML, Singh BK, Shaner DL (2005) Imidazolinone-tolerant crops: history, current status and future. Pest Manag Sci 61:246–257
Wang X, Wang H, Wang J, Sun R, Wu J, Liu S, Bai Y, Mun J-H, Bancroft I, Cheng F, Huang S, Li X, Hua W, Wang J, Wang X, Freeling M, Pires JC, Paterson AH, Chalhoub B, Wang B, Hayward A, Sharpe AG, Park B-S, Weisshaar B, Liu B, Li B, Liu B, Tong C, Song C, Duran C, Peng C, Geng C, Koh C, Lin C, Edwards D, Mu D, Shen D, Soumpourou E, Li F, Fraser F, Conant G, Lassalle G, King GJ, Bonnema G, Tang H, Wang H, Belcram H, Zhou H, Hirakawa H, Abe H, Guo H, Wang H, Jin H, Parkin IAP, Batley J, Kim J-S, Just J, Li J, Xu J, Deng J, Kim JA, Li J, Yu J, Meng J, Wang J, Min J, Poulain J, Hatakeyama K, Wu K, Wang L, Fang L, Trick M, Links MG, Zhao M, Jin M, Ramchiary N, Drou N, Berkman PJ, Cai Q, Huang Q, Li R, Tabata S, Cheng S, Zhang S, Zhang S, Huang S, Sato S, Sun S, Kwon S-J, Choi S-R, Lee T-H, Fan W, Zhao X, Tan X, Xu X, Wang Y, Qiu Y, Yin Y, Li Y, Du Y, Liao Y, Lim Y, Narusaka Y, Wang Y, Wang Z, Li Z, Wang Z, Xiong Z, Zhang Z (2011) The genome of the mesopolyploid crop species Brassica rapa. Nat Genet 43:1035–1039
Westerfeld W (1945) A colorimetric determination of blood acetoin. J Biol Chem 161:495–502
Yang LY, Liu PW, Yang GS (2006) Development of Polima temperature-sensitive cytoplasmic male sterile lines of Brassica napus through isolated microspore culture. Plant Breed 125:368–371
Yu C, Hu S, He P, Sun G, Zhang C, Yu Y (2006) Inducing male sterility in Brassica napus L. by a sulphonylurea herbicide, tribenuron-methyl. Plant Breed 125:61–64
Zhao MX, Du JC, Lin F, Tong CB, Yu JY, Huang SM, Wang XW, Liu SY, Ma JX (2013) Shifts in the evolutionary rate and intensity of purifying selection between two Brassica genomes revealed by analyses of orthologous transposons and relics of a whole genome triplication. Plant J 76:211–222
Acknowledgments
This work was supported by the National Hi-Tech R&D Program (2013AA102602), National Technology R&D Program (2012BAD49G00) and the Fundamental Research Funds for the Central Universities (Program No. 2662013YB03). GJK is a Chutian scholar, funded by Hubei province. We thank the two anonymous reviewers for their valuable suggestions.
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical standards
The authors declare that the experiments comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Isobel Parkin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, H., Li, J., Zhao, B. et al. Generation and characterization of tribenuron-methyl herbicide-resistant rapeseed (Brasscia napus) for hybrid seed production using chemically induced male sterility. Theor Appl Genet 128, 107–118 (2015). https://doi.org/10.1007/s00122-014-2415-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-014-2415-7