Abstract
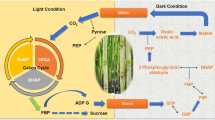
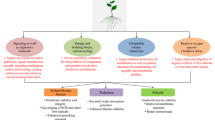
Sucrose is required for plant growth and development. The sugar status of plant cells is sensed by sensor proteins. The signal generated by signal transduction cascades, which could involve mitogen-activated protein kinases, protein phosphatases, Ca2+ and calmodulins, results in appropriate gene expression. A variety of genes are either induced or repressed depending upon the status of soluble sugars. Abiotic stresses to plants result in major alterations in sugar status and hence affect the expression of various genes by down- and up-regulating their expression. Hexokinase-dependent and hexokinase-independent pathways are involved in sugar sensing. Sucrose also acts as a signal molecule as it affects the activity of a proton-sucrose symporter. The sucrose transporter acts as a sucrose sensor and is involved in phloem loading. Fructokinase may represent an additional sensor that bypasses hexokinase phosphorylation especially when sucrose synthase is dominant. Mutants isolated on the basis of response of germination and seedling growth to sugars and reporter-based screening protocols are being used to study the response of altered sugar status on gene expression. Commoncis-acting elements in sugar signalling pathways have been identified. Transgenic plants with elevated levels of sugars/sugar alcohols like fructans, raffinose series oligosaccharides, trehalose and mannitol are tolerant to different stresses but have usually impaired growth. Efforts need to be made to have transgenic plants in which abiotic stress responsive genes are expressed only at the time of adverse environmental conditions instead of being constitutively synthesized.
Similar content being viewed by others
Abbreviations
- CDPK:
-
Calcium dependent protein kinase
- 2-DG:
-
2-deoxy glucose
- 6-DG:
-
6-deoxy glucose
- GA:
-
gibberellic acid
- G-6-P:
-
glucose-6-phosphate
- HSF:
-
heat shock transcription factor
- HXK:
-
hexokinase
- 3-o-MG:
-
3-oxy methyl glucose
- PC:
-
plastocyanin
- RFO:
-
raffinose family oligosaccharides
- SNF:
-
sucrose nonfermenting
- SPS:
-
sucrose phosphate synthase
- SRS:
-
sugar response sequence
- SuSy:
-
sucrose synthase
- TPS:
-
trehalose-6-phosphate synthase
References
Abebe T, Guenzi A C, Martin B and Cashman J C 2003 Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity;Plant Physiol. 131 1748–1755
Anderson C M and Kohorn B D 2001 Inactivation ofArabidopsis SIP1 leads to reduced levels of sugars and drought tolerance;J. Plant Physiol. 158 1215–1219
Arenas-Huertero F, Arroyo A, Zhou L, Sheen T and Leon P 2000 Analysis ofArabidopsis glucose insensitive mutants, gin 5 and gin 6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugars;Genes Dev. 14 2085–2086
Arroyo A, Bossi F, Finkelstein R R and Leon P 2003 Three genes that affect sugar sensing (Abscisic acid insensitive 4, Abscisic acid insensitive 5 and constitutive triple response 1) are differentially regulated by glucose inArabidopsis;Plant Physiol. 133 231–242
Atanssova R, Leterrier M, Gaillard C, Agasse A, Sagot F, Cou-tosaThevenot P and Delrot S 2003 Sugar-regulated expression of a putative hexose transport gene in grape;Plant Physiol. 131 326–334
Barker L, Kuhn C, Weise A, Schulz A, Gebhardt C, Hirner B, Hellman H, Schulze W, Ward J M and Frommer W B 2000SUT2, a putative sucrose sensor in sieve elements;Plant Cell 12 1153–1164
Briens M and Larher F 1983 Sorbitol accumulation in plantaginaceae: further evidence for functions in stress tolerance;Z. Pflanzenphysiol. 110 447–458
Brun T, Roche E, Kim K-H and Prentik M 1993 Glucose regulates acetyl CoA carboxylase gene expression in a pancreatic Β-cell line (INS-1);J. Biol. Chem. 268 18905–18911
Bustos M M, Iyer M and Gagliardi S J 1998 Induction of a Β-phaseolin promoter by exogenous abscisic acid in tobacco: Developmental regulation and modulation by external sucrose and Ca2+ ions;Plant Mol. Biol. 37 265–274
Castrillo M 1992 Sucrose metabolism in bean plants under water deficit;J. Exp. Bot. 43 1557–1561
Chan M T and Yu S M 1998a The 3 untranslated region of a rice α-amylase gene mediates sugar-dependent abundance of mRNA;Plant J. 15 685–695
Chan M T and Yu S M 1998b The 3 untranslated region of a rice α-amylase gene functions as a sugar-dependent mRNA stability determinant;Proc. Natl. Acad. Sci. USA 95 6543–6547
Cheng W H, Endo A, Zhou I, Penney J, Chen H C, Arroyo A, Leon P, Nambara E, Asami T and Seo S 2002 A unique short-chain dehydrogenase/reductase inArabidopsis abscisic acid biosynthesis and glucose signalling;Plant Cell 14 2723–2743
Chikano H, Ogawa M, Ikeda Y, Koizumi N, Kusano T and Sano H 2001 Two novel genes encoding SNF-1 related protein kinase fromArabidopsis thaliana: differential accumulation of AtSR1 and AtSR2 transcripts in response to cytokinins and sugars and phosphorylation of synthase by AtSR2;Mol. Gen. Genet. 264 674–681
Chiou T J and Bush D R 1998 Sucrose is a signal molecule in assimilate partitioning;Proc. Natl. Acad. Sci. USA 95 4784–4788
Coruzzi G M and Bush D R 2001 Nitrogen and carbon nutrient and metabolite signaling in plants;Plant Physiol. 125 61–64
Daie J 1988 Mechanism of drought-induced alterations in assimilate partitioning and transport in crops;CRC Crit. Rev. Plant Sci. 7 117–137
Demel R A, Dorrepaal E, Ebskamp M J M, Smeekens S and de Kruijff B 1998 Fructans interact strongly with model membranes;Biochim. Biophys. Acta 1375 36–42
Dijkwel P P, Huijser C, Weisbeek P J, Chua N-H and Smeekens S C M 1997 Sucrose control of phytochrome A signaling inArabidopsis;Plant Cell 9 583–595
Dijkwel P P, Kock P, Bezemer R, Weisbeek P and Smeekens S C M 1996 Sucrose represses the developmentally controlled transient activation of the plastocyanin gene inArabidopsis thaliana seedlings;Plant Physiol. 110 455–463
Dowrie B, Gurusinghe S, Dahal P, Thacker R R, Synhder J C, Nonogaki H, Yim K, Fukanaga K, Alvarado V and Bradford K J 2003 Expression of agalactinol synthase gene in tomato seeds is up-regulated before maturation desiccation and again after imbibition whenever radicle protrusion is prevented;Plant Physiol. 131 1347–1359
Du L and Chen Z 2000 Identification of genes encoding receptor-like protein kinases as possible target of pathogen- and salicylic acid-induced WRKY DNA-binding proteins inArabidopsis;Plant J. 24 837–847
Eastmond P J and Graham I A 2001 Re-examining the role of the glyoxylate cycle in oilseeds;Trends Plant Sci. 6 72–78
Ehness R, Ecker M, Godt D E and Roitsch T H 1997 Glucose and stress independently regulate source and sink metabolism and defence mechanisms via signal transduction pathways involving protein phosphorylation;Plant Cell 9 1825–1841
Everard J D, Gucci R, Kann S C, Flore J A and Loescher W H 1994 Gas exchange in the leaves of celery (Apium graveolens L.) at various levels of root zone salinity;Plant Physiol. 106 281–292
Fernie A R, Roessner U and Geigenberger P 2001 The sucrose analogue palatinose leads to a stimulation of sucrose degradation and starch synthesis when supplied to discs of growing potato tubers;Plant Physiol. 125 1967–1977
Foyer C H 1988 Feed back inhibition of photosynthesis through source-sink regulation in leaves;Plant Physiol. Biochem. 26 483–492
Fu H, Kim S Y and Park W D 1995 High level tuber expression and sucrose-inducibility of a potatoSus4 sucrose synthase gene requires 5 and 3 flanking sequences and the leader intron;Plant Cell 7 1387–1394
Gao M, Tao R, Miura K, Dandekar A M and Sugiura A 2001 Transformation of Japanese persimmon (Diospyros kaki Thunb) with apple cDNA encoding NADP-dependent sorbitol-6phosphate dehydrogenase;Plant Sci. 160 837–845
Garg A K, Kim J-K, Owens T G, Ranwala A P, Choi Y D, Kochian L V and Wu R J 2002 Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses;Proc. Natl. Acad. Sci. USA 99 15898–15903
Gazzarrini S and McCourt P 2001 Genetic interactions between ABA, ethylene and sugar signalling pathways;Curr. Opin. Plant Biol. 4 387–391
Godt D E and Roitsch T 1997 Differential regulation of a tomato invertase gene family suggests an important function of extracellular isoenzymes in establishing and maintaining sink metabolism;Plant Physiol. 115 273–282
Gupta A K and Kaur N 2000 Fructan metabolism in Jerusalem artichoke and chicory; inCarbohydrate reserves in plants — Synthesis and regulation (eds) A K Gupta and N Kaur (The Netherlands: Elsevier Science) pp 223–248
Gupta A K, Singh J, Kaur N and Singh R 1993a Effect of polyethylene glycol-induced water stress on uptake, interconversion and transport of sugars in chickpea seedlings;Plant Physiol. Biochem. 31 743–747
Gupta A K, Singh J, Kaur N and Singh R 1993b Effect of polyethylene glycol-induced water stress on germination and reserve carbohydrate metabolism in chickpea cultivars differing in tolerance to water deficit;Plant Physiol. Biochem. 31 369–378
Hare P D, Cress W A and van Staden J 1998 Dissecting the roles of osmolyte accumulation during stress;Plant Cell Environ. 21 535–554
Harrington N and Bush D R 2003 The bifunctional role of hexokinase in metabolism and glucose signaling;Plant Cell 15 2493–2496
Hattori T, Fukumoto H, Nakagawa S and Nakamura K 1991 Sucrose-induced expression of genes coding for tuberous root storage protein, sporamin, of sweet potato in leaves and petioles;Plant Cell Physiol. 32 79–86
Hincha D K, Hellwege E M, Heyer A G and Crowe J H 2000 Plant Fructans stabilize phosphatidylcholine liposomes during freeze-drying;Eur. J. Biochem. 267 535–540
Ho S-L, Chao Y-C, Tong W-F and Yu S-M 2001 Sugar coordinately and differentially regulates growthand stress-regulated gene expression via a complex signal transduction network and multiple control mechanisms;Plant Physiol. 125 877–890
Ho Y, Tang W, Swain J D, Green A L, Jack T P and Gan S 2001 Networking senescence-regulating pathways by usingArabidopsis enhancer trap lines;Plant Physiol. 126 707–716
Holmstrom K O, Mantyla E, Welin B, Mandal A, Palva E T, Tunnela O E and Londesborough J 1996 Drought tolerance in tobacco;Nature (London) 379 683–684
Huber S C, Huber J L, Liao P C, Gage D A and McMichael R W Jr 1996 Phosphorylation of serine-158 of maize leaf synthase. Occurrencein vivo and possible regulatory significance;Plant Physiol. 112 793–802
Hwang Y S, Karrer E E, Thomas B R, Chen L and Rodriguez R L 1998 Three cis-elements required for rice α-amylaseAmy3D expression during sugar starvation;Plant Mol. Biol. 36 331–341
Ingram J and Bartels D 1996 The molecular basis of dehydration tolerance in Plants;Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 377–403
Ishiguro S and Nakamura K 1994 Characterization of a cDNA encoding a novel DNA-binding protein, SPF1, that recognizes SP8 sequences in the 5 upstream region of genes coding for sporamin and Β-amylase from sweet potato;Mol. Gen. Genet. 28 563–571
Jang J C and Sheen J 1994 Sugar sensing in higher plants;Plant Cell 6 1665–1679
Jang J-C and Sheen J 1997 Sugar sensing in higher plants;Plant Cell 9 5–19
Jefferson R, Goldsbrough A and Bevan M 1990 Transcriptional regulation of apatatin-1 gene in potato;Plant Mol. Biol. 14 995–1006
Karakas B, Ozias-Akins P, Stushnoff C, Suefferheld M and Rieger M 1997 Salinity and drought tolerance of mannitolaccumulating transgenic tobacco;Plant Cell Environ. 20 609–616
Kaur P, Gupta A K and Kaur N 2005 Embryo is not required for initiation of α-amylase activity in germinating cowpea seeds;Indian J. Biochem. Biophys. 42 161–165
Kaur S, Gupta A K and Kaur N 2000 Effect of GA3, kinetin and indole acetic acid on carbohydrate metabolism in chickpea seedlings germinating under water stress;Plant Growth Regul. 30 61–70
Kaur S, Gupta A K and Kaur N 1998 Gibberellin A3 reverses the effect of salt stress in chickpea (Cicer arietinum L.) seedlings by enhancing the amylase activity and mobilization of starch in cotyledons;Plant Growth Regul. 26 85–90
Keller F and Ludlow M M 1993 Carbohydrate metabolism in drought stressed leaves of pigeon pea (Cajanus cajan);J. Exp. Bot. 44 1351–1359
Kim J Y, Mahe A, Brangeon J and Prioul J L 2000 A maize vacuolar invertase IVR2 is induced by water stress, organ tissue specificity and diurnal modulation of expression;Plant Physiol. 124 71–84
Kim K N and Guiltinam M J 1999 Identification of cis-acting elements important for expression of the starch-branching enzyme 1 gene in maize endosperm;Plant Physiol. 121 225–236
Kim S Y, May G D and Park W D 1994 Nuclear protein factors binding to class 1 patatin promoter region are tuber-specific and sucrose inducible;Plant Mol. Biol. 26 603–615
Koch K E 1996 Carbohydrate modulated gene expression in plants;Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 509–540
Koch K E, Nolte K D, Duke E R, McCarty D R and Avigne W T 1992 Sugar levels modulate differential expression of maize sucrose synthase genes;Plant Cell 4 59–69
Krapp A and Stitt M 1995 An evaluation of direct and indirect mechanisms for the sink-regulation of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady state transcript levels after cold-girdling source leaves;Planta 195 313–323
Kühn C, Barker L, Burkle L and Frommer W B 1999 Up date on sucrose transport in higher plants;J. Exp. Bot. 50 935–953
Kühn C, Franceschi V R, Schulz A, Lemoine R and Frommer W B 1997 Localization and turnover of sucrose transporters in enucleate sieve elements indicate macromolecular trafficking;Science 275 1298–1300
Kühn C, Quick W P, Schulz A, Riesmeier J W, Sonnewald V and Frommer W B 1996 Companion cell-specific in inhibition of the potato sucrose transporterSUT1;Plant Cell Environ. 19 1115–1123
Lalonde S, Boles E, Hellmann H, Barker L, Patrick J W, Frommer W B and Wood J M 1999 The dual action of sugar carriers: Transport and sugar sensing;Plant Cell 11 707–726
Liu J J, Krenz D C, Galvez A F and DeLumen B O 1998 Galactinol synthase (GS) increased enzyme activity and levels of mRNA due to cold and desiccation;Plant Sci. 134 11–20
Lopez-Molina L, Mongrand S and Chua N H 2001 A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the AB 15 transcription factor inArabidopsis;Proc. Natl. Acad. Sci. USA 98 4782–4787
Loreti E, Alpi A and Perata P 2000 Glucose and disaccharide sensing mechanisms modulate the expression of α-amylase in barley embryos;Plant Physiol. 123 939–948
Loreti E, Bellis L D, Alpi A and Perata P 2001 Why and how do plant cells sense sugars;Ann. Bot. 88 803–812
Lu C A, Lim E K and Yu S M 1998 Sugar response sequence in the promoter of a rice α-amylase gene serves as a transcriptional enhancer;J. Biol. Chem. 273 10120–10131
Maeo K, Tomiya T, Hayashi K, Akaike M, Morikami A, Ishiguro S and Nakamura K 2001 Suger-responsive elements in the promoter of a gene for Β-amylase of sweet potato;Plant Mol. Biol. 46 627–637
Maleck K, Levine A, Euglem T, Morgan A, Schmid J, Lawton K A, Dangi J L and Dietrich R A 2000 The transcriptome ofArabidopsis thaliana during systemic acquired resistance;Nat. Genet. 26 403–410
Martin T, Hellman H, Schmidt R, Willmitzer L and Frommer W B 1997 Identification of mutants in metabolically regulated gene expression;Plant J. 11 53–62
Martinez-Garcia J F, Huq E and Quali P H 2000 Direct targeting of light signals to a promoter element-bound transcriptional factor;Science 288 859–863
Meer I M, Ebskamp M J M, Visser R G F, Weisbeek P J and Smeekens S C M 1994 Fructan as a new carbohydrate sink in transgenic potato plants;Plant Cell 6 561–570
Minorsky P V 2003 The hot and the classic;Plant Physiol. 131 1159–1160
Mita S, Hirano H and Nakamura K 1997a Negative regulation in the expression of a sugar-inducible gene inArabidopsis thaliana; a recessive mutation causing enhanced expression of a gene for Β-amylase;Plant Physiol. 114 575–582
Mita S, Murano N, Akaike M and Nakamura K 1997b Mutants ofArabidopsis thaliana with pleiotropic effects on the expression of the gene for Β-amylase and on the accumulation of anthocyanin that are inducible by sugars;Plant J. 11 841–851
Mita S, Suzuki-Fujii K and Nakamura K 1995 Sugar inducible expression of a gene for Β-amylase inArabidopsis thaliana;Plant Physiol. 107 895–904
Moore B, Zhou L, Rolland F, Hall Q, Cheng W-H, Liu Y-X, Hwang I, Jones T and Sheen J 2003 Role ofArabidopsis glucose sensor HXK1 in nutrient, light and hormonal signalling;Science 300 332–336
Morita A, Umemura T, Kuroyanagi M, Futsuhara Y, Perata P and Yamaguchi J 1998 Functional dissection of a sugarrepressed α-amylase gene (Ramy1A) promo tor in rice embryos;FEBS Lett. 423 81–85
Nakamura K, Ohto M, Yoshida N and Nakamura K 1991 Sucrose-induced accumulation of Β-amylase occurs concomitant with the accumulation of starch and sporamin in leafpetiole cutting of sweet potato;Plant Physiol. 96 902–909
Nover L, Bharti K, Doring P, Mishra S K, Ganguli A and Scharf K D 2001Arabidopsis and the heat stress transcription factor world. How many heat stress transcription factors do we need?;Cell Stress Chaperones 6 177–189
Ohto M, Hayashi K, Isobe M and Nakamura K 1995 Involvement of Ca+2 signaling in the sugar-inducible expression of genes coding for sporamin and Β-amylase of sweet potato;Plant J. 7 297–307
Ohto M and Nakamura K 1995 Sugar-induced increase of calcium dependent protein kinases associataed with the plasma membrane in leaf tissues of tobacoo;Plant Physiol. 109 973–981
Ohto M, Nakamura-Kito K and Nakamura K 1992 Induction of expression of genes coding for sporamin and Β-amylase by polygalacturonic acid in leaf-petiole cuttings of sweet potato;Plant Physiol. 99 422–427
Panikulangara T J, Eggers-Schumacher G, Wunderlich M, Stransky H and Schöffl F 2004Galactinol synthase1. A novel heat shock factor target gene responsible for heat-induced synthesis of raffinose family oligosaccharides inArabidopsis;Plant Physiol. 136 3148–3158
Pego J and Smeekens S C M 2000 Plant fructokinases: a sweet family get-together;Trends Plant Sci. 5 531–536
Pelleschi S, Guy S, Kim J Y, Pointe C, Mahe A, Barthens L, Leonardi A and Prioul J L 1999IVR2, a candidate gene for a QTL of vacuolar invertase activity in maize leaves. Genespecific repression under water stress;Plant Mol. Biol. 39 373–380
Pelleschi S, Rocher J-P and Prioul J-L 1997 Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves;Plant Cell Environ. 20 493–503
Pennycooke J C, Jones M L and Stushnoff C 2003 Down-regulating α-galactosidase enhances freezing tolerance in transgenic potato;Plant Physiol. 133 1–9
Perata R T N, Williamson J D, Conkling M A and Pharr D M 1997 Sugar repression of mannitol dehydrogenase activity in celery cells;Plant Physiol. 114 307–314
Pilon-Smits E A H, Ebskamp M J M, Paul M J, Jeuken M J W, Weisbeek P J and Smeekens S C M 1995 Improved performance of transgenic fructan accumulating tobacco under drought stress;Plant Physiol. 107 125–130
Pilon-Smits E A H, Terry N, Sears T, Kim H and Van Dun K 1999 Enhanced drought resistance in fructan-producing sugar beet;Plant Physiol. Biochem. 37 313–317
Pilon-Smits E A H, Terry N, Sears T, Kim H, Zayed A, Hwang S, van Dun K, Voogd E, Verwoerd T C, Krutwagen R W and Goodijin O J M 1998 Trehalose producing transgenic tobacco plants show improved growth performance under drought stress;J. Plant Physiol. 152 525–532
Pla M, Vilardell J, Guiltinan M J, Marotte W R, Niogret M F, Quatrano R S and Pages M 1993 The cis-regulatory element CCACGTGG is involved in ABA and water-stress responses of the maize generap28;Plant Mol. Biol. 21 259–266
Price J, Laxmi A, Martin S K and Jang J-C 2004 Global transcription profiling reveals multiple sugar signal transduction mechanisms inArabidopsis;Plant Cell 16 2128–2150
Quick P, Siegl G, Neuhaus E, Feil R and Stitt M 1989 Short term water stress leads to a stimulation of sucrose synthesis by activating sucrose phosphate synthase;Planta 177 535–546
Rentsch D, Boorer K and Frommer W B 1998 Molecular biology of sucrose, amino acid and oligopeptide transporters at the plasma membrane of plant cells;J. Membr. Biol. 162 177–190
Riesmeier J W, Willmitzer L and Frommer W B 1994 Evidence for an essential role of sucrose transporter in phloem loading and assimilate partitioning;EMBO J. 13 1–7
Roitsch T 1999 Source-sink regulation by sugar and stress;Curr. Opin. Plant Biol. 2 198–206
Roitsch T, Balibrea H E, Hofmann M, Proels R and Sinha A K 2003 The upregulation of extracellular invertase was suggested to be a common response to various biotic and abiotic stress related stimuli like pathogen infection and salt stress;J.Exp.Bot. 54 513–524
Roitsch T, Bittner M and, Godt D E 1995 Induction of apoplastic invertase ofChenopodium rubrumby D-glucose and a glucose analogue and tissue-specific expression suggest a role in sink-source regulation;Plant Physiol. 108 285–294
Rolland F, Moore B and Sheen J 2002 Sugar sensing and signaling in plants;Plant Cell S185–S205
Rolland F, Winderickx J and Thevelein J M 2001 Glucose sensing mechanisms in eukaryotic cells;Trends Biochem. Sci. 26 310–317
Rook F, Gerrits N, Kortsee A, van Kampe M and Borrias M 1998 Sucrose-specific signaling represses translation of theArabidopsis ATB2 bZ1 transcription factor gene;Plant J. 15 253–263
Rushton P J, Macdonald H, Huttly A K, Lazarus C M and Hooley R 1995 Members of a new family of DNA-binding proteins bind to a conserved cis-element in the promoters of α-Amy2 genes;Plant Mol. Biol. 29 691–702
Sadaka A, De Wald D B, May G D, Park W D and Mullet J E 1994 Phosphate modulates transcription of soybeanVspB and other sugar inducible genes;Plant cell 6 737–749
Sarah C J, Graham I A, Reynolds S J, Leaver C J and Smith S M 1996 Distinct cis-acting elements direct the germination and sugar responses of the cucumber malate synthase genes;Mol. Gen. Genet. 250 153–161
Saravitz D M, Pharr D M and Carter T E 1987 Galactinol synthase activity and soluble sugars in developing seeds of four soybean genotypes;Plant Physiol. 83 185–189
Seki M, Narusaki M, Ishida J, Nanjo T, Fujita M, Oone Y, Kamiya A, Nakajima M, Enju A, Sakurai T, Satou M, Akiyama K, Taji T, Shinozaki K Y, Carninci P, Kawai J, Hayashizaki K Y and Shinozaki K 2002 Monitoring the expression profiles of 7000Arabidopsis genes under drought, cold and high salinity stresses using a full length cDNA micro array;Plant J. 31 279–292
Sevenier R, Hall R D, Meer I M, Hakkert H J C, Tunen A J and Koops A J 1998 High level fructan accumulation in a transgenic sugar beet;Nature Biotechnol. 16 843–846
Sheen J 1999 C4 gene expression;Annu. Rev. Plant Physiol. Plant Mol. Biol. 50 187–217
Shen B, Jensen R G and Bohnert H J 1997 Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts;Plant Physiol. 113 1177–1183
Sinha A K, Romer U, Kockenberger W, Hofmann M, Elling L and Roitsch T 2002 Metabolism and non-metabolizable sugars activate different signal transduction pathways in tomato;Plant Physiol. 128 1480–1489
Smeekens S 1998 Sugar regulation of gene expression in plants;Curr. Opin. Plant Biol. 1 230–234
Smeekens S 2000 Sugar induced signal transduction in plants;Annu. Rev. Plant Physiol. Plant Mol. Biol. 52 49–81
Smeekens S and Rook F 1997 Sugar sensing and sugar mediated signal transduction in plants;Plant Physiol. 115 7–13
Sprenger N and Keller F 2000 Allocation of raffinose family oligosaccharides to transport and storage pools inAjuga reptans: the role of two distinct galactinol synthases;Plant J. 21 249–258
Stitt M and Krappe A 1999 The interaction between elevated carbon dioxide and nitrogen nutrition. The physiological and molecular background;Plant Cell Environ. 22 583–621
Stoop J M H and Pharr D M 1994 Growth substrate and nutrient salt environment after mannitol to hexose partitioning in celery petioles;J. Am. Soc. Hortic. Sci. 119 237–242
Sturm A and Jang G-Q 1999 The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning;Trends Plant Sci. 4 401–407
Taji T, Qhsumi C, Iuchi S, Seki M, Kasuga M, Kobayashi M, Yamaguchi-Shinozaki K and Shinozaki K 2002 Important roles of drought-and cold-inducible genes for galactinol synthase in stress tolerance inArabidopsis thaliana;Plant J. 29 417–426
Tarczynski M C, Jensen R G and Bohnert H J 1992 Expression of a bacterial withmtlD gene in transgenic tobacco leads to production and accumulation of mannitol;Proc. Natl. Acad. Sci. USA 89 2600–2604
Tarczynski M C, Jensen R G and Bohnert H J 1993 Stress protection of transgenic plants by production of osmolyte mannitol;Science 259 508–510
Tegeder M, Wang X D, Frommer W B, Offler C E and Patric J W 1999 Sucrose transport into developing seeds ofPisum sativum L;Plant J. 18 151–161
Thomas J C, Sepathi M, Arendall B and Bohnert H J 1995 Enhancement of seed germination in high salinity by engineering mannitol expression inArabidopsis thaliana;Plant Cell. Environ. 18 801–806
Thomashow M F 1999 Plant cold accumulation: freezing tolerance genes and regulatory mechanisms;Annu. Rev. Plant. Physiol. Plant Mol. Biol. 50 571–599
Toroser D and Huber S C 1997 Protein phosphorylation as a mechanism for osmotic stress activation of sucrose-phosphate synthase in spinach leaves;Plant Physiol. 114 947–955
Umemura T, Perata P, Futsuhara Y and Yamaguchi J 1998 Sugar sensing and α-amylase gene expression in rice embryos;Planta 204 420–428
Urwin N A R and Jenkins G I 1997 A sucrose repression element in thePhaseolus vulgaris rbcS2 gene promoter resembles responsible for sugar stimulation of plant and mammalian genes;Plant Mol. Biol. 35 929–942
Vereyken I J, Chupin V, Demel R A, Smeekens S and de Kruijff B 2001 Fructans insert between the headgroups of phospholipids;Biochim. Biophys. Acta 1510 307–320
Wanner L A and Junttila O 1999 Cold-induced freezing tolerance inArabidopsis;Plant Physiol. 120 391–399
Ward J, Kühn C, Tegeder M and Frommer W B 1998 Sucrose transport in plants;Int. Rev. Cytol. 178 41–47
Weber H, Borisjuk L, Heim U, Sauter N and Wobus U 1997 A role for sugar transporters during seed development, molecular characterization of a hexose and a sucrose carrier in fava bean seeds;Plant Cell 9 895–908
Weschke W, Paritz R, Saner N, Wang Q, Neubohn B, Weber H and Wobus U 2000 Sucrose transport into barley seeds: Molecular characterization of two transporters and implications for seed development and starch accumulation;Plant J. 21 455–467
Wu K, Rooney M F and Ferl R J 1997 TheArabidopsis 14-3-3 multigene family;Plant Physiol. 114 1421–1431
Xiao W, Sheen J and Jang J-C 2000 The role of hexokinase in plant sugar signal transduction and growth and development;Plant Mol. Biol. 44 451–461
Yeo E T, Kwon H B, Han S E, Lee J T, Ryn J C and Byun M O 2000 Genetic engineering of drought-resistant potato plants by introduction of the trehalose-6-phosphate synthase (TPS1) gene fromSaccharomyces cerevisae;Mol. Cells 10 263–268
Yokohama R, Hirosa T, Fuju N, Aspuria E T, Kats A and Uchimiya H 1997 The rolC promotor ofAgrobacterium rhizogenes Ri plasmid is activated by sucrose in transgenic tobacco plants;Mol. Gen. Genet. 244 15–22
Zhao H W, Chen Y J, Hu Y L, Gao Y and Lin Z P 2000 Construction of a trehalose-6-phosphate synthase gene driven by drought-responsive promotor and expression of droughtresistance in transgenic tobacco;Acta Bot. Sinica 42 616–619
Zhou L, Jang J C, Jones T L and Sheen J 1998 Glucose and ethylene signal transduction cross talk revealed by anArabidopsis glucose-insensitive mutant;Proc. Natl. Acad. Sci. USA 95 10294–10299
Zinselmeier C, Westgate M E, Schussler J R and Jones R J 1995 Low water potential disrupts carbohydrate metabolism in maize (Zea mays L.) ovaries;Plant Physiol. 107 385–391
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, A.K., Kaur, N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 30, 761–776 (2005). https://doi.org/10.1007/BF02703574
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02703574