Abstract
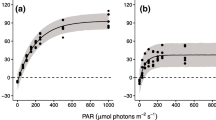
The time-course of photoadaptation in the symbiotic sea anemone Anemonia viridis (Forskäl) was determined on freshly collected anemones which were maintained under low light (LL: 10 μE m-2s-1) or high light (HL: 300 μE m-2s-1) over a period of 35 d. Photosynthesis versus irradiance curves (P vs I) normalised to anemone dry weight were determined for anemones at the start of the experiment and for both groups at 7 d intervals. Photosynthetic parameters derived from the P vs I curves, P grossmax (the maximum photosynthetic capacity of the zooxanthellae), α (an estimate of photosynthetic efficiency) and I k(the light-saturating irradiance), and the respiration rate, showed that anemones were high-light adapted before the experiment began. Thereafter, any observed changes occurred only in anemones adapting to the low-light regime. P grossmax remained relatively constant over 35 d and there was no significant difference between LL and HL groups. In the LL group α increased and both I kand the respiration rate decreased significantly. These photoadaptive responses were most rapid in the first 7 d, after which change was gradual until about Day 28, when photoadaptation appeared to be complete. In order to predict the physiological mechanisms of photoadaptation to low light, P gross vs I curves normalised to zooxanthellae biomass were constructed for LL and HL anemones at 35 d. Zooxanthellae density and photosynthetic pigment content were also analysed. For LL-adapted anemones, α and chlorophyll a were higher, there were no differences in algal density and chlorophyll c 2 and I kwas lower. It is proposed that the size of the peridinin-chlorophyll a-protein complex (PCP) of the light-harvesting complex was increased to facilitate photoadaptation. Plotting P net vs I normalised to anemone dry weight on the 35 d-photoadapted anemones gave a measure of changes in the excess photosynthetically fixed carbon that was potentially available to the host. Net photosynthesis of the LL group was higher at subsaturating light intensities as a result of the increase in α, and at saturating light intensities P netmax was higher as a result of a decrease in the rate of respiration of the whole symbiotic association.
Similar content being viewed by others
References
Achituv Y, Dubinsky Z (1990) The adaptation of symbiotic associations between zooxanthellae and corals at different light levels. In: Lesel R (ed) Microbiology in poecilotherms. Elsevier Science Publishers, BV, Amsterdam, pp 43–46
Al-Sofyani, Davies PS (1994) Seasonal variation in production and respiration in Red Sea corals. Proc 7th int coral Reef Symp [Richmond RH (ed) University of Guam in Mangilao, Guam] (in press)
Battey JF, Porter JW (1989) Photoadaptation as a whole organism response in Montastrea annularis. Proc 6th int coral Reef Symp 3:79–86 [Choat JH, et al (eds) Sixth International Coral Reef Symposium Executive Committee, Townsville]
Chalker BE (1981) Simulating light-saturation curves for photosynthesis and calcification by reef-building corals. Mar Biol 63: 135–141
Chalker BE, Dunlap WC, Oliver JK (1983) Bathymetric adaptations of reef building corals at Davies Reef, Great Barrier Reef, Australia. II. Light saturation curves for photosynthesis and respiration. J exp mar Biol Ecol 73:37–56
Chang SS, Prézelin BB, Trench RK (1983) Mechanisms of photoadaptation in three strains of the symbiotic dinoflagellate Symbiodinium microadriaticum. Mar Biol 76:219–229
Clayton WS, Lasker HR (1984) Host feeding regime and zooxanthellal photosynthesis in the anemone Aiptasia pallida (Verrill). Biol Bull mar biol Lab, Woods Hole 167:590–600
Davies PS (1977) Carbon budgets and vertical zonation of Atlantic reef corals. Proc 3rd int coral Reef Symp 1:391–396 [Taylor DL (ed) Rosenstiel School of Marine and Atmospheric Science, University of Miami]
Davies P (1980) Respiration in some Atlantic reef corals in relation to vertical distribution and growth form. Biol Bull mar biol Lab, Woods Hole 158:187–194
Davies PS (1990) A rapid method for assessing growth rates of corals in relation to water pollution. Mar Pollut Bull 21:346–348
Davies PS (1991) Effect of daylight variations on energy budgets of shallow-water corals. Mar Biol 108:137–144
Dustan P (1979) Distribution of zooxanthellae and photosynthetic chloroplast pigments of the reef-building coral Montastrea annularis, Ellis and Solander, in relation to depth on a West Indian reef. Bull mar Sci 29:79–95
Edmunds PJ, Davies PS (1988) Post-illumination stimulation of respiration rate in the coral Porites porites. Coral Reefs 7:7–9
Edmunds PJ, Davies PS (1989) An energy budget for Porites porites (Scleractinia) growing in a stressed environment. Coral Reefs 8:37–43
Falkowski PG (1990) Irradiance and corals. In: Dubinsky Z (ed) Coral reefs. Elsevier Science Publishers BV, Amsterdam, pp 89–107
Falkowski PG, Dubinsky Z (1981) Light-shade adaptation of Stylophora pistillata, a hermatypic coral from the Gulf of Eilat. Nature, Lond 289:172–174
Gattuso JP (1985) Features of depth effects on Stylophora pistillata, an hermatypic coral in the Gulf of Aqaba (Jordan, Red Sea). Proc 5th int coral Reef Congr 6:95–100 [Gabrié C, et al. (eds) Antenne Museum-EPHE, Moorea, French Polynesia]
Green EJ, Carritt DE (1967) New tables for oxygen saturation of sea water. J mar Res 25:140–147
Harland AD, Fixter LM, Davies PS, Anderson RA (1992) Effect of light on the total lipid content and storage lipids of the symbiotic sea anemone Anemonia viridis. Mar Biol 112:253–258
Hoegh-Guldberg O, Smith GJ (1989) Influence of the population density of zooxanthellae and supply of ammonium on the biomass and metabolic characteristics of the reef corals Seriatopora hystrix and Stylophora pistillata. Mar Ecol Prog Ser 57:173–186
Iriarte A, Purdie D (1993) Photosynthesis and growth response of the oceanic picoplankter Pycnococcus provasolii Guillard (clone ω48–23) (Chlorophyta) to variations in irradiance, photoperiod and temperature. J exp mar Biol Ecol 168:239–257
Janssen HH, Möller H (1981) Effects of various feeding conditions on Anemonia sulcata. Zool Anz 206:161–170
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, c, c 1 and c 2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pfl 167: 191–194
Muller-Parker G (1985) Effect of feeding regime and irradiance on the photophysiology of the symbiotic sea anemone Aiptasia pulchella. Mar Biol 90:65–74
Muller-Parker G (1987) Seasonal variation in light-shade adaptation of natural populations of the symbiotic sea anemone Aiptasia pulchella (Carlgren, 1943) in Hawaii. J exp mar Biol Ecol 112:165–183
Muscatine L, Falkowski PG, Dubinsky Z, Cook PA, McCloskey LR (1989) The effect of external nutrient resources on the population dynamics of zooxanthellae in a reef coral. Proc R Soc (Ser B) 236:311–324
Muscatine L, Falkowski PG, Porter JW, Dubinsky Z (1984) Fate of photosynthetic carbon in light- and shade-adapted colonies of Stylophora pistillata. Proc R Soc (Ser B) 222:181–202
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for sea water analysis. Pergamon Press, Oxford
Porter JW, Muscatine L, Dubinsky Z, Falkowski PG (1984) Primary production and photoadaptation in light- and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proc R Soc (Ser B) 222:161–180
Prézelin BB (1987) Photosynthetic physiology of dinoflagellates. In: Taylor FJR (ed) The biology of the dinoflagellates. Blackwell Scientific Publications, Oxford, p 174–223
Prézelin BB, Ley AC, Haxo FT (1976) Effect of growth irradiance on the photosynthetic action spectra of the marine dinoflagellate Glenodinium sp. Planta 130:251–256
Prézelin BB, Matlick HA (1983) Nutrient-dependent low-light adaptation in the dinoflagellate Gonyaulax polyedra. Mar Biol 74: 141–150
Prézelin BB, Sweeney BM (1978) Photoadaptation of photosynthesis in two bloom-forming dinoflagellates. In: Taylor DL, Seliger HH (eds) Toxic dinoflagellate blooms. Elsevier North Holland, New York, pp 101–106
Sokal RR, Rohlf FJ (1981) Biometry. The principles and practice of statistics in biological research. 2nd ed. Freeman WH & Co, San Francisco
Steele RD (1976) Light intensity as a factor in the regulation of the density of symbiotic zooxanthellae in Aiptasia tagetes (Coelenterata, Anthozoa). J Zool, Lond 179:387–405
Svoboda A, Porrmann T (1980) Oxygen production and uptake by symbiotic Aiptasia diaphana (Rapp) (Anthozoa, Coelenterata) adapted to different light intensities. In: Smith DC, Tiffon Y (eds) Nutrition in the lower metazoa. Pergamon Press, New York, pp 87–99
Thinh L-V (1991) Photo-adaption in two species of Acropora grown under controlled conditions. Photosynthetica 25:365–371
Titlyanov EA, Shaposhnikova MG, Zvalinski VI (1980) Photosynthesis and adaptation of corals to irradiance. I. Contents and native state of photosynthetic pigments in symbiotic microalga. Photosynthetica 14:413–421
Trench RK (1987) Dinoflagellates in non-parasitic symbiosis. In: Taylor FJR (ed) The biology of the dinoflagellates. Blackwell Scientific Publications, Oxford, pp 530–570
Tytler EM (1982) The contribution of zooxanthellae to the energy requirements of the sea anemone anemonia sulcata (Pennant). PhD thesis. University of Glasgow
Tytler EM, Davies PS (1984) Photosynthetic production and respiratory energy expenditure in the anemone Anemonia sulcata (Pennant). J exp mar Biol Ecol 81:73–86
Wethey DS, Porter JW (1976) Sun and shade differences in productivity of reef corals. Nature, Lond 262:281–282
Zvalinski VI, Letekin VA, Titlyanov EA, Shaposhnikova MG (1980) Photosynthesis and adaptation of corals to irradiance. II. Oxygen exchange. Photosynthetica 14:422–430
Author information
Authors and Affiliations
Additional information
Communicated by J. Mauchline, Oban
Rights and permissions
About this article
Cite this article
Harland, A.D., Davies, P.S. Time-course of photoadaptation in the symbiotic sea anemone Anemonia viridis . Marine Biology 119, 45–51 (1994). https://doi.org/10.1007/BF00350105
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00350105