Abstract
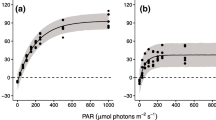
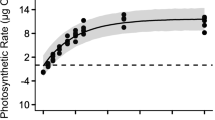
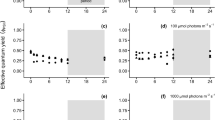
This study examined the effects of temperature and irradiance on photosynthetic characteristics of the macroscopic sporophyte (SPO) and microscopic gametophyte (GAM) stages of a subarctic brown alga, Saccharina japonica var. japonica (Laminariales) from Hokkaido, Japan. In vitro measurements under short- and long-term exposures were carried out by using optical dissolved oxygen sensors and the pulse amplitude modulation (PAM)-chlorophyll fluorometer, respectively. The heteromorphic life history stages of S. japonica showed photosynthetic optima at 23–23.3 °C, derived from the gross photosynthesis–temperature model. Maximum quantum yields (Fv/Fm) of SPO and GAM after 72 h of temperature exposures in the dark were reduced to near zero above 24 °C, indicating PSII inactivation. Such similarity in their temperature characteristics suggests the co-occurrence of both generations in the habitat despite the seasonal growth and reproduction of the species. Net photosynthesis–irradiance experiments in the two life history stages at 8, 16, and 24 °C revealed similarity in their light-saturated photosynthetic rates (NPmax = 3.02–4.41 μg O2 gww−1 min−1, SPO; 2.87–3.73 μg O2 gww−1 min−1, GAM), but saturation irradiances of SPO (Ek = 81–102 μmol photons m−2 s−1) were higher than those of GAM (48–69 μmol photons m−2 s−1). A slight decrease in net photosynthetic rates of GAM above 500 μmol photons m−2 s−1 was likewise observed. This difference may be related to the light regime of their natural habitat, suggesting the low irradiance adaptation of the microscopic stage that settles on rock crevices beneath algal canopies.



Similar content being viewed by others
References
Agatsuma Y, Endo H, Yoshida S, Ikemori C, Takeuchi Y, Fujishima H, Nakajima K, Sano M, Kanezaki N, Imai H, Yamamoto N, Kanahama H, Matsubara T, Takahashi S, Isogai T, Taniguchi K (2014) Enhancement of Saccharina kelp production by nutrient supply in the Sea of Japan off southwestern Hokkaido, Japan. J Appl Phycol 26:1845–1852
Alexandrov GA, Yamagata Y (2007) A peaked function for modeling temperature dependence of plant productivity. Ecol Model 200:189–192
Allakhverdiev SI, Kreslavski V, Klimov V, Los D, Carpentier R, Mohanty P (2008) Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res 98:541–550
Augyte S, Yarish C, Neefus CD (2019) Thermal and light impacts on the early growth stages of the kelp Saccharina angustissima (Laminariales, Phaeophyceae). Algae 34:153–162
Bartsch I, Wiencke C, Bischof K, Buchholz CM, Buck BH, Eggert A, Feuerpfeil P, Hanelt D, Jacobsen S, Karez R, Karsten U, Molis M, Roleda MY, Schubert H, Schumann R, Valentin K, Weinberger F, Wiese J (2008) The genus Laminaria sensu lato: recent insights and developments. Eur J Phycol 43:1–86
Beer S, Björk M, Beardall J (2014) Photosynthesis in the marine environment. Wiley & Sons, Ames, p 208
Bischof K, Buschbaum C, Fredriksen S, Gordillo FJL, Heinrich S, Jiménez C, Lütz C, Molis M, Roleda MY, Schwanitz M, Wiencke C (2019) Kelps and environmental changes in Kongsfjorden: stress perception and responses. In: Hop H, Wiencke C (eds) The ecosystem of Kongsfjorden, Svalbard. Springer, Cham, pp 373–422
Bixler HJ, Porse H (2011) A decade of change in the seaweed hydrocolloids industry. J Appl Phycol 23:321–335
Boo SM, Ko YD (2012) Marine plants from Korea. Junghaeng-Sa, Seoul, p 233 (in Korean)
Borlongan IA, Matsumoto K, Nakazaki Y, Shimada N, Kozono J, Nishihara GN, Shimada S, Watanabe Y, Terada R (2018) Photosynthetic activity of two life history stages of Costaria costata (Laminariales, Phaeophyceae) in response to PAR and temperature gradient. Phycologia 57:159–168
Borlongan IA, Nishihara GN, Shimada S, Terada R (2019a) Assessment of photosynthetic performance in the two life history stages of Alaria crassifolia (Laminariales, Phaeophyceae). Phycol Res 67:28–38
Borlongan IA, Maeno Y, Kozono J, Endo H, Shimada S, Nishihara GN, Terada R (2019b) Photosynthetic performance of Saccharina angustata (Laminariales, Phaeophyceae) at the southern boundary of distribution in Japan. Phycologia 58:300–309
Bürkner PC (2017) brms: an R package for Bayesian multilevel models using Stan. J Stat Softw 80:1–28
Bürkner PC (2018) Advanced Bayesian multilevel modeling with the R package brms. R J 10:395–411
Carney LT (2011) A multispecies laboratory assessment of rapid sporophyte recruitment from delayed kelp gametophytes. J Phycol 47:244–251
Carney LT, Edwards MS (2010) Role of nutrient fluctuations and delayed development in gametophyte reproduction by Macrocystis pyrifera (Phaeophyceae) in Southern California. J Phycol 46:987–996
Colombo-Pallotta MF, Rodríguez-Román A, Iglesias-Prieto R (2010) Calcification in bleached and unbleached Montastraea faveolata: evaluating the role of oxygen and glycerol. Coral Reefs 29:899–907
Cosgrove J, Borowitzka MA (2011) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and developments. Springer, Dordrecht, pp 1–17
Delebecq G, Davoult D, Janquin MA, Oppliger LV, Menu D, Dauvin JC, Gévaert F (2016) Photosynthetic response to light and temperature in Laminaria digitata gametophytes from two French populations. Eur J Phycol 51:71–82
Eggert A (2012) Seaweed responses to temperature. In: Wiencke C, Bischof K (eds) Seaweed biology. Springer, Berlin, pp 47–66
Fredersdorf J, Müller R, Becker S, Wiencke C, Bischof K (2009) Interactive effects of radiation, temperature and salinity on different life history stages of the Arctic kelp Alaria esculenta (Phaeophyceae). Oecologia 160:483–492
Fu G, Liu J, Wang G, Yao J, Wang X, Duan D (2010) Early development of Costaria costata (C. Agardh) Saunders and cultivation trials. Chin J Oceanol Limnol 28:731–737
Fukumoto R, Borlongan IA, Nishihara GN, Endo H, Terada R (2018) The photosynthetic responses to PAR and temperature including chilling-light stress on the heteromorphic life history stages of a brown alga, Cladosiphon okamuranus (Chordariaceae) from Ryukyu Islands, Japan. Phycol Res 66:209–217
Fukumoto R, Borlongan IA, Nishihara GN, Endo H, Terada R (2019) Effect of photosynthetically active radiation and temperature on the photosynthesis of two heteromorphic life history stages of a temperate edible brown alga, Cladosiphon umezakii (Chordariaceae, Ectocarpales), from Japan. J Appl Phycol 31:1259–1270
Gao X, Endo H, Agatsuma Y (2015) Effect of increased seawater temperature on biomass, growth, and maturation of Saccharina japonica near its southern limit in northern Japan. J Appl Phycol 27:1263–1270
Gao X, Endo H, Nagaki M, Agatsuma Y (2017) Interactive effects of nutrient availability and temperature on growth and survival of different size classes of Saccharina japonica (Laminariales, Phaeophyceae). Phycologia 56:253–260
Gelman A (2004) Parameterization and Bayesian modeling. J Am Stat Assoc 99:537–545
Gelman A (2006) Prior distributions for variance parameters in hierarchical models. Bayesian Anal 1:515–533
Hanelt D, Roleda MY (2009) Acclimation to UVB radiation may induce a reduction in photoinhibitory stress on some Caribbean marine macrophytes. Aquat Bot 91:6–12
Hanelt D, Wiencke C, Karsten U, Nultsch W (1997) Photoinhibition and recovery after high light stress in different developmental and life-history stages of Laminaria saccharina (Phaeophyta). J Phycol 33:387–395
Hanelt D, Wiencke C, Bischof K (2003) Photosynthesis in marine macroalgae. In: Larkum AW, Douglas SE, Raven JA (eds) Photosynthesis in algae. Kluwer Academic Publishers, Dordrecht, pp 413–435
Hasegawa Y (1971) Forced cultivation of Laminaria. Bull Hokkaido Regional Fish Res Lab 37:49–52
Henkel SK, Hofmann GE (2008) Thermal ecophysiology of gametophytes cultured from invasive Undaria pinnatifida (Harvey) Suringar in coastal California harbors. J Exp Mar Biol Ecol 367:164–173
Henley WJ (1993) Measurement and interpretation of photosynthetic light-response curves in algae in the context of photoinhibition and diel changes. J Phycol 29:729–739
Izquierdo J, Pérez-Ruzafa I, Gallardo T (2002) Effect of temperature and photon fluence rate on gametophytes and young sporophytes of Laminaria ochroleuca Pylaie. Helgol Mar Res 55:285–292
Japan Fisheries Resource Conservation Association (1996) Kelp. Japan Fisheries Resource Conservation Association, Tokyo, p 11 (in Japanese)
Japan Oceanographic Data Center (2020) JODC data on-line service system. http://jdoss1.jodc.go.jp/vpage/coastal_j.html. Accessed 13 Mar 2020 (in Japanese)
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Kawai T (2012) Saccharina. In: Watanabe MM, Inouye I, Okino T, Kamiya M, Kaya K, Kawai H, Kawachi M, Kusumi T, Shiraiwa Y (eds) Handbook of algae: their diversity and utilization. NTS, Tokyo, pp 590–597 (in Japanese)
Kawashima S (1993) Cultivation of the brown alga, Laminaria “Kombu”. In: Ohno M, Critchley AS (eds) Seaweed cultivation and marine ranching. Japan International Cooperation Agency (JICA), Yokosuka, pp 25–40
Kawashima S (2012) Morphology and taxonomy of the Laminariaceous algae in cold water area of Japan. Seibutsu-Kenkyusha, Tokyo, p 560 (in Japanese)
Kim JK, Yarish C, Hwang EK, Park M, Kim YD (2017) Seaweed aquaculture: cultivation technologies, challenges and its ecosystem services. Algae 32:1–13
Li JY, Murauchi Y, Ichinomiya M, Agatsuma Y, Taniguchi K (2007) Seasonal changes in photosynthesis and nutrient uptake in Laminaria japonica (Laminariaceae; Phaeophyta). Aquacult Sci 55:587–597
Li X, Pang SJ, Shan TF (2017) Genetic diversity and population structure among cultivars of Saccharina japonica currently farmed in northern China. Phycol Res 65:111–117
Liu F, Pang SJ (2010) Performances of growth, photochemical efficiency, and stress tolerance of young sporophytes from seven populations of Saccharina japonica (Phaeophyta) under short-term heat stress. J Appl Phycol 22:221–229
Mathur S, Agrawal D, Jajoo A (2014) Photosynthesis: response to high temperature stress. J Photechnol Photobiol B 137:116–126
McHugh DJ (2003) A guide to the seaweed industry, FAO Fisheries technical paper – T441. FAO, Rome
Mizuta H, Maita Y, Kuwada K (1994) Nitrogen recycling mechanism within the thallus of Laminaria japonica (Phaeophyceae) under the nitrogen limitation. Fish Sci 60:763–767
Mizuta H, Hayasaki J, Yamamoto H (1998) Relationship between nitrogen content and sorus formation in the brown alga Laminaria japonica cultivated in southern Hokkaido, Japan. Fish Sci 64:909–913
Müller R, Wiencke C, Bischof K (2008) Interactive effects of UV radiation and temperature on microstages of Laminariales (Phaeophyceae) from the Arctic and North Sea. Clim Res 37:203–213
Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta 1767:414–421
Ohno M (2004) Edible local seaweeds. In: Ohno M (ed) Biology and technology of economic seaweeds. Uchida Rokakuho Publishing, Tokyo, pp 283–296
Oppliger LV, Correa JA, Engelen AH, Tellier F, Vieira V, Faugeron S, Valero M, Gomez G, Destombe C (2012) Temperature effects on gametophyte life-history traits and geographic distribution of two cryptic kelp species. PLoS One 7:e39289
Pang SJ, Jin ZH, Sun JZ, Gao SQ (2007) Temperature tolerance of young sporophytes from two populations of Laminaria japonica revealed by chlorophyll fluorescence measurements and short-term growth and survival performances in tank culture. Aquaculture 262:493–503
Pereira L (2016) Edible seaweeds of the world. CRC Press, Boca Raton, p 463
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
R Development Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. Accessed 11 Apr 2020
Roleda MY (2009) Photosynthetic response of Arctic kelp zoospores exposed to radiation and thermal stress. Photobiol Sci 8:1302–1312
Roleda MY (2016) Stress physiology and reproductive phenology of Arctic endemic kelp Laminaria solidungula J Agardh. Polar Biol 39:1967–1977
Roleda MY, Wiencke C, Hanelt D, van de Poll WH, Gruber A (2005) Sensitivity of Laminariales zoospores from Helgoland (North Sea) to ultraviolet and photosynthetically active radiation: implications for depth distribution and seasonal reproduction. Plant Cell Environ 28:466–479
Roleda MY, Wiencke C, Hanelt D, Bischof K (2007) Sensitivity of the early life stages of macroalgae to ultraviolet radiation. Photochem Photobiol 83:851–862
Sakanishi Y, Iizumi H (2001) Photosynthetic responses to light in summer sporophytes of cold water species of Laminariales from the eastern coast of Hokkaido. Jap J Phycol 49:1–6
Sakanishi Y, Suzuki K, Udagawa T, Iizumi H, Yamamoto M (2001) The daily compensation depth for Laminaria longissima in summer along the coast of Kushiro, Hokkaido. Bull Hokk Natl Fish Res Inst 65:45–54
Salvucci ME, Crafts-Brandner SJ (2004) Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiol 134:1460–1470
Sasaki M (2017) The current status of Saccharina fishery in Hokkaido. Hokusuishi Dayori 94:5–9 (in Japanese)
Sato Y, Kozono J, Nishihara GN, Terada R (2020) Effect of light and temperature on photosynthesis of a cultivated brown alga, Saccharina sculpera (Laminariales), from Japan. Phycologia 59:375–384
Sharkey TD (2005) Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ 28:269–277
Steneck R, Graham MH, Bourque BJ, Corbertt D, Erlandson JM (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459
Swanson AK, Druehl LD (2000) Differential meiospore size and tolerance of ultraviolet light stress within and among kelp species along a depth gradient. Mar Biol 136:657–664
Tatewaki M (1966) Formation of a crustose sporophyte with unilocular sporangia in Scytosiphon lomentaria. Phycologia 6:62–66
Team SD (2020) RStan: the R interface to Stan. R package version 2.17.3. http://mc-stan.org. Accessed 11 Apr 2020
Terada R, Abe M, Abe T, Aoki M, Masakazu A, Dazai A, Endo H, Kamiya M, Kawai H, Kurashima A, Motomura T, Murase N, Sakanishi Y, Shimabukuro H, Tanaka J, Yoshida G, Aoki MA (2019) Japan’s nationwide long-term monitoring survey of seaweed communities known as the “Monitoring Sites 1000”: ten-year overview and future perspectives. Phycol Res. https://doi.org/10.1111/pre.12395
Terada R, Nakashima Y, Borlongan IA, Shimabukuro H, Kozono J, Endo H, Shimada S, Nishihara GN (2020) Photosynthetic activity including the thermal- and chilling-light sensitivities of a temperate Japanese brown alga Sargassum macrocarpum. Phycol Res 68:70–79
Thornley JHM, Johnson IR (2000) Plant and crop modelling: a mathematical approach to plant and crop physiology. Blackburn Press, Caldwell, p 669
Titlyanov EA, Titlyanov TV (2012) Marine plants of the Asian Pacific region countries, their use and cultivation. Dalnauka and A.V. Zhirmunsky Institute of Marine Biology, Far East Branch of the Russian Academy of Sciences, Vladivostok p 376 (in Russian with English description)
Tokida J (1954) The marine algae of southern Saghalin. Mem Fac Fish Hok Univ 1:1–264
tom Dieck I (1993) Temperature tolerance and survival in darkness of kelp gametophytes (Laminariales, Phaeophyta): ecological and biogeographic implications. Mar Ecol Prog Ser 100:253–264
Vásquez JAJ, Zuñiga S, Tala F, Piaget N, Rodríguez DC, Vega JMA (2014) Economic valuation of kelp forests in northern Chile: values of goods and services of the ecosystem. J Appl Phycol 26:1081–1088
Vásquez-Elizondo RM, Enríquez S (2016) Coralline algal physiology is more adversely affected by elevated temperature than reduced pH. Sci Rep 6:19030
Wang Y, Xu D, Fan X, Zhang X, Ye N, Wang W, Mao Y, Mou S, Cao S (2013) Variation of photosynthetic performance, nutrient uptake, and elemental composition of different generations and different thallus parts of Saccharina japonica. J Appl Phycol 25:631–637
Watanabe Y, Nishihara GN, Tokunaga S, Terada R (2014) The effect of irradiance and temperature on the photosynthesis of a cultivated red alga, Pyropia tenera (=Porphyra tenera), at the southern limit of distribution in Japan. Phycol Res 62:187–196
Watanabe Y, Yamada H, Takayuki M, Yoshio K, Nishihara GN, Terada R (2016) Photosynthetic responses of Pyropia yezoensis f. narawaensis (Bangiales, Rhodophyta) to a thermal and PAR gradient vary with the life-history stage. Phycologia 55:665–672
Webb WL, Newton M, Starr D (1974) Carbon dioxide exchange of Alnus rubra: a mathematical model. Oecologia 17:281–291
Wiencke C, Bartsch I, Bischoff B, Peters AF, Breeman AM (1994) Temperature requirements and biogeography of Antarctic, Arctic and amphiequatorial seaweeds. Bot Mar 37:247–259
Wiencke C, Clayton MN, Schoenwaelder M (2004) Sensitivity and acclimation to UV radiation of zoospores from five species of Laminariales from the Arctic. Mar Biol 145:31–39
Wiencke C, Lüder UH, Roleda MY (2007) Impact of ultraviolet radiation on physiology and development of zoospores of the brown alga Alaria esculenta from Spitsbergen. Physiol Plant 130:601–612
Yoshida T (1998) Marine algae of Japan. Uchida Rokakuho Publishing, Tokyo p 1222 (in Japanese)
Yotsukura N, Kawashima S, Kawai T, Abe T, Druehl LD (2008) A systematic re-examination of four Laminaria species: L. japonica, L. religiosa, L. ochotensis and L. diabolica. J Jpn Bot 83:165–176
Yuan XC, Zhao WW (2015) China Fishery Statistical Yearbook, Fishery Bureau, Ministry of Agriculture. China Agriculture Press, Beijing, pp 29–56 (in Chinese)
Zemke-White WL, Ohno M (1999) World seaweed utilization: an end-of-century summary. J Phycol 11:369–376
Zhang QS, Tang XX, Cong YZ, Qu SC, Luo SJ, Yang GP (2007) Breeding of an elite Laminaria variety through inter-specific gametophyte crossing. J Appl Phycol 19:303–311
Zhang QS, Qu SC, Cong YZ, Luo SJ, Tang XX (2008) High throughput culture and gametogenesis induction of Laminaria japonica gametophyte clones. J Appl Phycol 20:205–211
Zhang J, Liu Y, Yu D, Song H, Cui J, Liu T (2011) Study on high- temperature-resistant and high-yield Laminaria variety “Rongfu”. J Appl Phycol 23:165–171
Acknowledgments
We express our gratitude to Prof. Taizo Motomura and Prof. Chikako Nagasato of Muroran Marine Station, Hokkaido University, for their kind arrangement of our fieldwork in the study site. We also thank our lab mates, Ryo Kameyama, Tomohiro Ito, Jumpei Kozono, Masahiro Niibo, Kazuya Matsumoto, Yoshiki Nakazaki, Yumi Kyoda, and Yuki Watanabe, for their help in the experiments including the preliminary study. This field survey was conducted in collaboration with the nationwide long-term monitoring survey for seaweed communities (Monitoring Site 1000) of the Japanese Ministry of Environment. All authors have provided consent.
Funding
This research was supported in part by the Grant-in-Aid for Scientific Research (#26241027 and #16H02939) from the Japan Society for the Promotion of Science (JSPS) and the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Borlongan, I.A., Arita, R., Nishihara, G.N. et al. The effects of temperature and irradiance on the photosynthesis of two heteromorphic life history stages of Saccharina japonica (Laminariales) from Japan. J Appl Phycol 32, 4175–4187 (2020). https://doi.org/10.1007/s10811-020-02266-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02266-2