Abstract
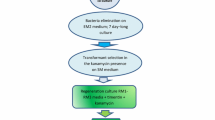
Epicotyl segments and nodus expiants from etiolated seedlings of Pisum sativum were transformed using Agrobacterium tumefaciens strains GV 2260 (p35S GUS INT) and GV 3850 HPT carrying either a neomycin- or hygromycinphosphotransferase-gene as selectable markers. The transgenic character of hygromycin- or kananamycin-resistant tissue was confirmed by detection of nopaline or neomycinphosphotransferase-II- and ß-glucuronidase activity in crude extracts of resistant tissues. Up to 5 % of developing shoots from shoot proliferating nodi were regenerated via organogenesis to kanamycin-resistant plantlets. Transformation frequency in vitro was found to be influenced by expiant source, A. tumefaciens strain, pea genotype and duration of cocultivation. Acetosyringone did not increase the transformation rate.
Similar content being viewed by others
Abbreviations
- GUS:
-
ß-glucuronidase
- NAA:
-
1-naphthyl-acetic-acid
- BA:
-
6-benzylaminopurine
- NPT-II:
-
neomycinphosphotransferase-II
- HPT:
-
hygromycinphosphotransferase
References
Bercetche J, Chriqui D, Adam S, David C (1987) Plant Science 52: 195–210
Bertoni G, Mills D (1987) Phytopathology 77: 832–835
Bradford MM (1976) Anal Biochem 72: 248–254
Broekart D, van Parijs R (1973) J Exp Bot 24: 820–827
Byrne MC, McDonnell RE, Wright MS, Carnes MG (1987) Plant Cell Tiss Organ Cult 8: 3–15
Chabaud M, Passiatore JE, Cannon F, Buchanan-Wollaston V (1988) Plant Cell Rep 7: 512–516
Deblaere R, Bytebier B, De Greve H, Deboeck F, Schell J, Van Montagu M, Leemans J (1985) Nucleic Acids Res 13: 4777–4788
Eapen S, Köhler, F, Gerdemann M, Schieder O (1987) Theor Appl Genet 75: 201–210
Gasser CS, Fraley RT (1989) Science 244: 1293–1299
Griga M, Tejklova E, Novak FJ, Kubalakova M (1986) Plant Cell Tiss Organ Cult 6: 95–104
Hinchee MAW, Connor-Ward DV, Newell CA, McDonnell RE, Sato SJ, Gasser CS, Fischhoff DA, Re DB. Fraley RT, Horsch RB (1988) Biotechnology 6: 915–921
Hobbs SLA, Jackson JA, Manon JD (1989) Plant Cell Rep 8: 274–277
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA (1983) Nature 303: 179–180
Hood EE, Helmer GL, Fraley RT, Chilton M-D (1986) J Bacteriol 168: 1291–1301
Hussey G, Johnson RD, Warren S (1989) Protoplasma 148: 101–105
Jefferson RA, Kavanagh TA, Bevan MW (1987) EMBO J 6: 3901–3907
Kodama A (1975) Japan J Genet 50: 291–299
Kurkdijan A, Manigault P, Beardsley R (1969) Can J Bot 47: 803–808
Kysely W, Myers JR, Lazzeri PA, Collins GB, Jacobsen H-J (1987) Plant Cell Rep 6: 305–308
Lehminger-Mertens R, Jacobsen H-J (1989) Plant Cell Rep 8: 379–382
Lopatin MI (1936) Microbiologia (Moskwa) 5: 716–724
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular Cloning: a laboratory manual, Cold Spring Harbor Laboratory, New York
Mariotti D, Fontana GS, Santini L (1989) J. Genet & Breed 43: 77–82
McHughen A, Jordan MC (1989) Plant Cell Rep 7: 611–61
Otten LABM, Schilperoort RA (1978) Biochim Biophys Acta 527: 497–500
Otten L, Piotrowiak G, Hooykaas PJJ, Dubois M, Szegedi E, Schell J (1985) Mol Gen Genet 199: 189–193
Parrott WA, Hoffman LM, Hildebrand DF, Williams EG, Collins GB (1989) Plant Cell Rep 7: 615–617
Polito VS, McGranahan G, Pinney K, Leslie C (1989) Plant Cell Rep 8: 219–221
Puonti-Kaerlas J, Stabel P, Eriksson T (1989) Plant Cell Rep 8: 321–324
Reiss B, Sprengel R, Will H, Schaller H (1984) Gene 30: 211–218
Smith EF, Townsend CO (1907) Science 25: 671–673
Stachel SE, Messens E, Van Montagu M, Zambryski P (1985) Nature 318: 624–629
Vancanneyt G, Schmidt R, O'Connor-Sanchez A, Willmitzer L, Rocha-Sosa (1990) Mol Gen Genet 220: 245–250
Zambryski P, Joos H, Genetello C, Leemans J, van Montagu M, Schell J (1983) EMBO J. 2: 2143–2150
Author information
Authors and Affiliations
Additional information
Communicated by H. Lörz
Rights and permissions
About this article
Cite this article
De Kathen, A., Jacobsen, HJ. Agrobacterium tumefaciens-mediated transformation of Pisum sativum L. using binary and cointegrate vectors. Plant Cell Reports 9, 276–279 (1990). https://doi.org/10.1007/BF00232301
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00232301