Abstract
Background
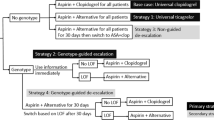
Results from the PROVE IT trial suggest that patients with acute coronary syndrome (ACS) treated with atorvastatin 80 mg/day (A80) have significantly lower rates of cardiovascular events compared with patients treated with pravastatin 40 mg/day (P40). In a genetic post hoc substudy of the PROVE IT trial, the rate of event reduction was greater in carriers of the Trp719Arg variant in kinesin family member 6 protein (KIF6) than in noncarriers. We assessed the cost effectiveness of testing for the KIF6 variant followed by targeted statin therapy (KIF6 Testing) versus not testing patients (No Test) and treating them with P40 or A80 in the USA from a payer perspective.
Methods
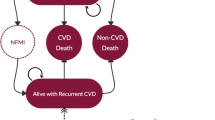
A Markov model was developed in which 2-year event rates from PROVE IT were extrapolated over a lifetime horizon. Costs and utilities were derived from published literature. All costs were in 2010 US dollars except the cost of A80, which was in 2012 US dollars because the generic formulation was available in 2012. Expected costs and quality-adjusted life-years (QALYs) were estimated for each strategy over a lifetime horizon.
Results
Lifetime costs were US$31,700; US$37,100 and US$41,300 for No Test P40, KIF6 Testing and No Test A80 strategies, respectively. The No Test A80 strategy was associated with more QALYs (9.71) than the KIF6 Testing (9.69) and No Test P40 (9.57) strategies. No Test A80 had an incremental cost-effectiveness ratio (ICER) of US$232,100 per QALY gained compared with KIF6 Testing. KIF6 Testing had an ICER of US$45,300 per QALY compared with No Test P40.
Conclusions
Testing ACS patients for KIF6 carrier status may be a cost-effective strategy at commonly accepted thresholds. Treating all patients with A80 is more expensive than treating patients on the basis of KIF6 results, but the modest gain in QALYs is achieved at a cost/QALY that is generally considered unacceptable compared with the KIF6 Testing strategy. Compared with treating all patients with P40, the KIF6 Testing strategy had an ICER below US$50,000 per QALY. The conclusions from this study are sensitive to the price of generic A80 and the effect on adherence of knowing KIF6 carrier status. The results were based on a post hoc substudy of the PROVE IT trial, which was not designed to test the effectiveness of KIF6 testing.






Similar content being viewed by others
References
Roger VL, Go AS, Lloyd-Jones DM, American Heart Association Statistics Committee and Stroke Statistics Subcommittee, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–209.
Schwartz GG, Olsson AG, Ezekowitz MD, Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285(13):1711–8.
Heart Disease and Stroke Statistics, 2009 update at a glance. Dallas: American Heart Association; 2009.
Kolansky DM. Acute coronary syndromes: morbidity, mortality, and pharmacoeconomic burden. Am J Manag Care. 2009;15(2 Suppl):S36–41.
Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504.
Iakoubova OA, Sabatine MS, Rowland CM, et al. Polymorphism in KIF6 gene and benefit from statins after acute coronary syndromes: results from the PROVE IT-TIMI 22 study. J Am Coll Cardiol. 2008;51:449–55.
Iakoubova OA, Tong CH, Rowland CM, et al. Association of the Trp719Arg polymorphism in kinesin-like protein 6 with myocardial infarction and coronary disease in 2 prospective trials: the CARE and WOSCOPS trials. J Am Coll Cardiol. 2008;51:435–43.
de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292(11):1307–16.
Li Y, Iakoubova OA, Shiffman D, et al. KIF6 polymorphism as a predictor of risk of coronary events and of clinical event reduction by statin therapy. Am J Cardiol. 2010;106(7):994–8.
Shroufi A, Powles JW. Adherence and chemoprevention in major cardiovascular disease: a simulation study of the benefits of additional use of statins. J Epidemiol Community Health. 2010;64(2):109–13.
Taylor DCA, Pandya A, Thompson D, et al. Cost-effectiveness of intensive atorvastatin therapy in secondary cardiovascular prevention in the United Kingdom, Spain and Germany, based on the Treating to New Targets study. Eur J Health Econ. 2009;10:255–65.
Lampe FC, Whincup PH, Wannamethee SG, et al. The natural history of prevalent ischaemic heart disease in middle-aged men. Eur Heart J. 2000;21(13):1052–62.
Data on file. Celera, 2011.
DrugReimbursement.com (online database). Eden Prairie (MN): Optum; 2012. http://www.drugreimbursement.com/drc/. Accessed 2 Jun 2012.
Drug topics red book 2010. Montvale (NJ): Thomson PDR; 2010.
O’Sullivan AK, Rubin J, Nyambose J, et al. Cost estimation of cardiovascular disease events in the US. Pharmacoeconomics. 2011;29(8):693–704.
Arias E. United States life tables 2006. Natl Vital Stat Rep. 2010;58(21):1–40.
Sullivan PW, Lawrence WF, Ghushchyan V. A national catalog of preference-based scores for chronic conditions in the United States. Med Care. 2005;43:736–49.
Kalia NK, Miller LG, Nasir K, et al. Visualizing coronary calcium is associated with improvements in adherence to statin therapy. Atherosclerosis. 2006;185(2):394–9.
Charland SL, Agatep BC, Epstein RS, et al. Patient knowledge of pharmacogenetic information improves adherence to statin therapy: results of the additional KIF6 risk offers better adherence to statins (AKROBATS) trial. J Am Coll Cardiol. 2012;59(13):E1848.
Nelson AL, Cohen JT, Greenberg D, et al. Much cheaper, almost as good: decrementally cost-effective medical innovation. Ann Intern Med. 2009;151:662–7.
Parris ES, Lawrence DB, Mohn LA, et al. Adherence to statin therapy and LDL cholesterol goal attainment by patients with diabetes and dyslipidemia. Diabetes Care. 2005;28(3):595–9.
Assimes TL, Hólm H, Kathiresan S, et al. Lack of association between the Trp719Arg polymorphism in kinesin-like protein-6 and coronary artery disease in 19 case-control studies. (J Am Coll Cardiol. 2010 Nov 2, 56(19), pp. 1552–63. Erratum in). J Am Coll Cardiol. 2011;57(4):520.
Fung G, Aparicio AF, Wu A, Smith A, Wong I. KIF6 carrier status in Asians predicts beneficial response to statins. Circulation. 2012;125:AP327.
Ridker PM, MacFadyen JG, Glynn RJ, et al. Kinesin-like protein 6 (KIF6) polymorphism and the efficacy of rosuvastatin in primary prevention. Circ Cardiovasc Genet. 2011;4(3):312–7.
Hopewell JC, Parish S, Clarke R, et al. No impact of KIF6 genotype on vascular risk and statin response among 18,348 randomized patients in the Heart Protection Study. J Am Coll Cardiol. 2011;57(20):2000–7.
Ford I, Murray H, Packard CJ, et al. Long-term follow-up of the West of Scotland coronary prevention study. N Engl J Med. 2007;357(15):1477–86.
Ference BA, Yoo W, Flack JM, Clarke M. A common KIF6 polymorphism increases vulnerability to low-density lipoprotein cholesterol: two meta-analyses and a meta-regression analysis. PLoS ONE. 2011;6(12):e28834.
Iakoubova OA, Robertson M, Tong CH, et al. KIF6 Trp719Arg polymorphism and the effect of statin therapy in elderly patients: results from the PROSPER study. Eur J Cardiovasc Prev Rehabil. 2010;17(4):455–61.
Acknowledgments
This study was sponsored by the Celera Corporation, Alameda, CA, USA. Anju Parthan, Kevin Leahy and Amy O’Sullivan are employees of OptumInsight, Cambridge, MA, and were paid consultants to Celera in connection with the development of the manuscript. Milton Weinstein was a paid consultant to OptumInsight in connection with the development of this manuscript. Olga Iakoubova, Lance Bare and James Devlin are full-time employees of Celera, a wholly-owned subsidiary of Quest Diagnostics. Olga Iakoubova and James Devlin are inventors on patents related to the KIF6 719Arg variants. Berkeley HeartLab, a wholly-owned subsidiary of Quest Diagnostics, offers a KIF6 genotyping test, which is a laboratory-developed test that was developed and validated pursuant to the Clinical Laboratory Improvement Amendments (CLIA).
Author contributions
Anju Parthan, Kevin J. Leahy, Amy K. O’Sullivan, Olga A. Iakoubova, Lance A. Bare, James J. Devlin and Milton C. Weinstein participated in the design of the model, the interpretation of data and results, and the drafting of the manuscript. Anju Parthan will serve as a guarantor for the overall content of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parthan, A., Leahy, K.J., O’Sullivan, A.K. et al. Cost Effectiveness of Targeted High-dose Atorvastatin Therapy Following Genotype Testing in Patients with Acute Coronary Syndrome. PharmacoEconomics 31, 519–531 (2013). https://doi.org/10.1007/s40273-013-0054-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-013-0054-5