Abstract
Introduction
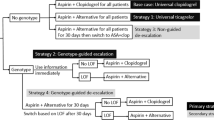
The POPular Genetics trial demonstrated that a CYP2C19 genotype-guided P2Y12 inhibitor strategy reduced bleeding rates compared with standard treatment with ticagrelor or prasugrel without increasing thrombotic event rates after primary percutaneous coronary intervention (PCI).
Objective
In this analysis, we aimed to evaluate the cost effectiveness of a genotype-guided strategy compared with standard treatment with ticagrelor or prasugrel.
Methods
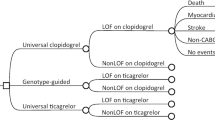
A 1-year decision tree based on the POPular Genetics trial in combination with a lifelong Markov model was developed to compare costs and quality-adjusted life-years (QALYs) between a genotype-guided and a standard P2Y12 inhibitor strategy in patients with myocardial infarction undergoing primary PCI. The cost-effectiveness analysis was conducted from a Dutch healthcare system perspective. Within-trial survival and utility data were combined with lifetime projections to evaluate lifetime cost effectiveness for a cohort of 1000 patients. Costs and utilities were discounted at 4 and 1.5%, respectively, according to Dutch guidelines for health economic studies. Besides deterministic and probabilistic sensitivity analyses, several scenario analyses were also conducted (different time horizons, different discount rates, equal prices for P2Y12 inhibitors, and equal distribution of thrombotic events between the two strategies).
Results
Base-case analysis with a hypothetical cohort of 1000 subjects demonstrated 8.98 QALYs gained and €725,550.69 in cost savings for the genotype-guided strategy (dominant). The deterministic and probabilistic sensitivity analysis confirmed the robustness of the model and the cost-effectiveness results. In scenario analyses, the genotype-guided strategy remained dominant.
Conclusion
In patients undergoing primary PCI, a CYP2C19 genotype-guided strategy compared with standard treatment with ticagrelor or prasugrel resulted in QALYs gained and cost savings.
Trial Registration
Clinicaltrials.gov number: NCT01761786, Netherlands trial register number: NL2872



Similar content being viewed by others
References
Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68:1082–115.
Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–77.
Scott SA, Sangkuhl K, Shuldiner AR, et al. PharmGKB summary: very important pharmacogene information for cytochrome P450, family 2, subfamily C, polypeptide 19. Pharmacogenet Genomics. 2012;22:159–65.
Scott SA, Sangkuhl K, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94:317–23.
Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–30.
Holmes DR, Dehmer GJ, Kaul S, Leifer D, O’Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology foundation task force on clinical export consensus documents and the American Heart Association endorsed by the Society of Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2010;56:321–41.
Motovska Z, Hlinomaz O, Kala P, et al. 1-year outcomes of patients undergoing primary angioplasty for myocardial infarction treated with prasugrel versus ticagrelor. JACC. 2018;71:371–81.
Gimbel ME, Qaderdan K, Willemsen L, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. 2020;395:1374–81.
Zettler ME, Peterson ED, McCoy LA, et al. Switching of adenosine diphosphate receptor inhibitor after hospital discharge among myocardial infarction patients: insights from the treatment with adenosine diphosphate receptor inhibitors: Longitudinal assessment of treatment patterns and events after acute coronary syndrome (TRANSLATE-ACS) observational study. Am Heart J. 2017;183:62–8.
Claassens DMF, Vos GJA, Bergmeijer TO, et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med. 2019;381:1621–31.
Bergmeijer TO, Janssen PWA, Schipper JC, et al. CYP2C19 genotype-guided antiplatelet therapy in ST-segment elevation myocardial infarction patients-Rationale and design of the patient outcome after primary PCI (POPular) Genetics study. Am Heart J. 2014;168:16-22.e.1.
Nikolic E, Janzon M, Hauch O, Wallentin L, Henriksson M. Cost-effectiveness of treating acute coronary syndrome patients with ticagrelor for 12 months: results from the PLATO study. Eur Heart J. 2012;34:220–8.
Central Bureau for Statistics. Lifetables. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/83482NED/table?ts=1603871419734. Accessed 15 Oct 2020.
Lala A, Berger JS, Sharma G, Hochman JS, Braithwaite RS, Ladapo JA. Genetic testing in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a cost-effectiveness analysis. J Thromb Haemost. 2013;11:81–91.
Zorginstituut Nederland. GIPdatabank: Vergoeding per DDD 2015-2019 voor ATC-subgroep B01AC04 : Clopidogrel. https://www.gipdatabank.nl/databank?infotype=g&label=00-totaal&tabel_g_00-totaal=B_01-basis&geg=vg&spec=vg_ddd&item=B01AC04. Accessed 15 Oct 2020.
Zorginstituut Nederland. GIPdatabank: Vergoeding per DDD 2015-2019 voor ATC-subgroep B01AC24 : Ticagrelor. https://www.gipdatabank.nl/databank?infotype=g&label=00-totaal&tabel_g_00-totaal=B_01-basis&geg=vg&spec=vg_ddd&item=B01AC24. Accessed 15 Oct 2020.
Zorginstituut Nederland. GIPdatabank: Vergoeding per DDD 2015-2019 voor ATC-subgroep B01AC22 : Prasugrel. https://www.gipdatabank.nl/databank?infotype=g&label=00-totaal&tabel_g_00-totaal=B_01-basis&geg=vg&spec=vg_ddd&item=B01AC22. Accessed 15 Oct 2020.
Jacobs MS, De Jong LA, Postma MJ, Tieleman RG, Van Hulst M. Health economic evaluation of rivaroxaban in elective cardioversion of atrial fibrillation. Eur J Health Econ. 2018;19:957–65.
ten Cate-Hoek AJ, Toll DB, Buller HR, et al. Cost-effectiveness of ruling out deep venous thrombosis in primary care versus care as usual. J Thromb Haemost. 2009;7:2042–9.
Stevanovic J, Pompen M, Le HH, Rozenbaum MH, Tieleman RG, Postma MJ. Economic evaluation of apixaban for the prevention of stroke in non-valvular atrial fibrillation in the Netherlands. PLoS ONE. 2014;9:e103974.
De Jong LA, Groeneveld J, Stefanovic J, et al. Cost-effectiveness of apixaban compared to other anticoagulants in patients with atrial fibrillation in the real-world and trial settings. PLoS ONE. 2019;14(9):e0222658.
Van Eeden M, Van Heugten C, Van Mastrigt GAPG, Van Mierlo M, Visser-Meily JMA, Evers SMAA. Evers SMAA. The burden of stroke in the Netherlands: estimating quality of life and costs for 1 year poststroke. BMJ Open. 2015;5:e008220.
Ijzerman MJ, Al MJ, De Boer A, et al. Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg. Zorginstituut Ned. 2016; 1–38. https://www.zorginstituutnederland.nl/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg. Accessed 15 Oct 2020.
Ligtenberg G, Staal PC, Goettsch WG, Knies S. Kosteneffectiviteit in de zorg, op weg naar een genuanceerd en geaccepteerd gebruik van kosteneffectiviteitsgegevens in de zorg. College voor Zorgverzekeringen 2013; 1–41. https://www.zorginstituutnederland.nl/binaries/zinl/documenten/rapport/2013/09/30/kosteneffectiviteit-in-de-zorg/Kosteneffectiviteit+in+de+zorg.pdf. Accessed 15 Oct 2020.
Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304–22.
Wang Y, Yan BP, Liew D, Lee VWY. Cost-effectiveness of cytochrome P450 2C19 *2 genotype-guided selection of clopidogrel or ticagrelor in Chinese patients with acute coronary syndrome. Pharmacogenom J. 2018;18:113–20.
Limdi NA, Cavallari LH, Lee CR, et al. Cost-effectiveness of CYP2C19-guided antiplatelet therapy in patients with acute coronary syndrome and percutaneous coronary intervention informed by real-world data. Pharmacogenom J. 2020;20:724–35.
Sorich MJ, Horowitz JD, Sorich W, Wiese MD, Pekarsky B, Karnon JD. Cost-effectiveness of using CYP2C19 genotype to guide selection of clopidogrel or ticagrelor in Australia. Pharmacogenomics. 2013;14:2013–21.
Crespin DJ, Federspiel JJ, Biddle AK, Jonas DE, Rossi JS. Ticagrelor versus genotype-driven antiplatelet therapy for secondary prevention after acute coronary syndrome: a cost-effectiveness analysis. Value Health. 2011;14:483–91.
Mahoney EM, Wang K, Arnold SV, et al. Cost-effectiveness of prasugrel versus clopidogrel in patients with acute coronary syndromes and planned percutaneous coronary intervention: results from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction TRITON-TIMI 38. Circulation. 2010;121:71–9.
Schüpke S, Neumann FJ, Menichelli M, et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. 2019;381:1524–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by ZonMw, a Dutch government agency, as part of its efficiency research program (project 171102022). Spartan Bioscience (Nepean, Ottawa, ON, Canada) provided the Spartan RX system and the reagents used free of charge.
Conflicts of interest
Dr. Asselbergs has received grants from the University College London Hospitals National Institute for Health Research Biomedical Research Centre. Dr. van 't Hof has received institutional grants from Medtronic, AstraZeneca, and Sanofi and personal fees from AstraZeneca and Amgen. Dr. Barbato has received personal fees from Boston Scientific, Abbott Vascular, and GE. Dr. Ten Berg has received institutional grants from AstraZeneca and ZonMw and personal fees from AstraZeneca, Daiichi Sankyo, Eli Lilly, the Medicines Company, Accumetrics, Boehringer-Ingelheim, Bayer, BMS, Pfizer, and Ferrer. Dr. Postma has received institutional grants from AstraZeneca, GSK, Pfizer, and Amgen and personal fees from AstraZeneca, Daiichi Sankyo, Boehringer-Ingelheim, Bayer, BMS, Pfizer, GSK, and Roche. Daniel M.F. Claassens, Pim W.M. van Dorst, Gerrit J.A. Vos, Thomas O. Bergmeijer, Renicus S. Hermanides, Pim van der Harst, Carmine Morisco, Richard M. Tjon Joe Gin, Arend Mosterd, Jean-Paul R. Herrman, Willem J.M. Dewilde, Vera H.M. Deneer, and Cornelis Boersma have no conflicts of interest that are directly relevant to the content of this article.
Availability of data and material
Data will be made available upon reasonable request
Code availability
Analyses were performed using R version 3.6 or later and Excel.
Ethics approval
An institutional review board at each site approved the trial.
Consent to participate
All patients provided informed consent to participate in the trial.
Author contributions
Conceptualization: TOB, JMtB, VHMD. Methodology: DMFC, WMvD, CB. Formal analysis and investigation: DMFC, WMvD, TOB, GJAV. Writing—original draft preparation: DMFC, WMvD. Writing—review and editing: GJAV, TOB, RSH, AWJv’tH, PvdH, EB, CM, RMTJG, FWA, AM, JPRH, WJMD, MJP. Funding acquisition: VHMD. Resources: RSH, AWJv’tH, PvdH, EB, CM, RMTJG, FWA, AM, JPRH, WJMD. Supervision: VHMD, JMtB, CB, MJP.
Rights and permissions
About this article
Cite this article
Claassens, D.M.F., van Dorst, P.W.M., Vos, G.J.A. et al. Cost Effectiveness of a CYP2C19 Genotype-Guided Strategy in Patients with Acute Myocardial Infarction: Results from the POPular Genetics Trial. Am J Cardiovasc Drugs 22, 195–206 (2022). https://doi.org/10.1007/s40256-021-00496-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-021-00496-4