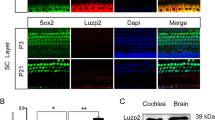
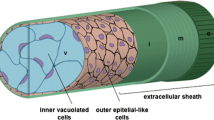
Abstract
The mammalian cochlea is a highly intricate organ responsible for hearing. Numerous specialized cell types residing in the cochlear participate in processing and relaying sound information to the brain. In general, cells in the cochlea are divided into three major types: sensory, neural, and non-sensory. Sensory cells are a group of cells in the organ of Corti consisting of hair cells and supporting cells. Sensory hair cells play a primary role in detecting and processing sound in the form of vibrations. Neural cells are the neurons and glia in the spiral (cochlear) ganglion that relay the processed sound signals in the form of a neurotransmitter to the brain. Other non-sensory cells include all other cell types providing architectural and functional support. Building a functional cochlea requires tightly orchestrated, spatial and temporal regulation of gene expressions. Disruption of the normal gene expression patterns can cause developmental failure of the organ, which can lead to permanent hearing loss. Thus, comprehensive understanding of genes contributing to cochlear development is crucial for elucidating the pathological mechanisms of hearing loss. This article is intended to provide an overview of mammalian cochlear development, focusing on genes involved in its early patterning.
Similar content being viewed by others
References
Ahn S and Joyner AL (2004) Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118: 505–516.
Alsina B, Giraldez F and Pujades C (2009) Patterning and cell fate in ear development. Int. J. Dev. Biol. 53: 1503–1513.
Bok J, Bronner-Fraser M and Wu DK (2005) Role of the hindbrain in dorsoventral but not anteroposterior axial specification of the inner ear. Development 132: 2115–2124.
Bok J, Brunet LJ, Howard O, Burton Q and Wu DK (2007a) Role of hindbrain in inner ear morphogenesis: Analysis of Noggin knockout mice. Dev. Biol. 311: 69–78.
Bok J, Chang W and Wu DK (2007b) Pattering and morphogenesis of the vertebrate inner ear. Int. J. Dev. Biol. 51: 521–533.
Bok J, Dolson DK, Hill P, Ruther U, Epstein DJ and Wu DK (2007c) Opposing gradients of Gli repressor and activators mediate Shh signaling along the dorsoventral axis of the inner ear. Development 134: 1713–1722.
Braunstein EM, Crenshaw Iii EB, Morrow BE and Adams JC (2008) Cooperative Function of Tbx1 and Brn4 in the Periotic Mesenchyme is Necessary for Cochlea Formation. J. Assoc. Res. Otolaryngol. 9: 33–43.
Chang W, ten Dijke P and Wu DK (2002) BMP pathways are involved in otic capsule formation and epithelial-mesenchymal signaling in the developing chicken inner ear. Dev. Biol. 251: 380–394.
Chen P, Johnson JE, Zoghbi HY and Segil N (2002) The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129: 2495–2505.
Chen P and Segil N (1999) p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 126: 1581–1590.
Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH and Kelley MW (2008) Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc. Natl. Acad. Sci. USA 105: 18396–18401.
Delprat B, Ruel J, Guitton MJ, Hamard G, Lenoir M, Pujol R, Puel JL, Brabet P and Hamel CP (2005) Deafness and cochlear fibrocyte alterations in mice deficient for the inner ear protein otospiralin. Mol. Cell Biol. 25: 847–853.
Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK and Segil N (2009) Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev. Cell. 16: 58–69.
Driver EC, Pryor SP, Hill P, Turner J, Ruther U, Biesecker LG, Griffith AJ and Kelley MW (2008) Hedgehog Signaling Regulates Sensory Cell Formation and Auditory Function in Mice and Humans. J. Neurosci. 28: 7350–7358.
Fekete DM and Wu DK (2002) Revisiting cell fate specification in the inner ear. Curr. Opin. Neurobiol. 12: 35–42.
Frenz DA and Van De Water TR (1991) Epithelial control of periotic mesenchyme chondrogenesis. Dev. Biol. 144: 38–46.
Hayashi T, Cunningham D and Bermingham-McDonogh O (2007) Loss of Fgfr3 leads to excess hair cell development in the mouse organ of Corti. Dev. Dyn. 236: 525–533.
Hayashi T, Ray CA and Bermingham-McDonogh O (2008) Fgf20 is required for sensory epithelial specification in the developing cochlea. J. Neurosci. 28: 5991–5999.
Jacob J and Briscoe J (2003) Gli proteins and the control of spinal-cord patterning. EMBO Rep. 4: 761–765.
Jacques BE, Montcouquiol ME, Layman EM, Lewandoski M and Kelley MW (2007) Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development 134: 3021–3029.
Jones C and Chen P (2007) Planar cell polarity signaling in vertebrates. Bioessays 29: 120–132.
Kelley MW (2006) Regulation of cell fate in the sensory epithelia of the inner ear. Nat. Rev. Neurosci. 7: 837–849.
Kelley MW, Driver EC and Puligilla C (2009) Regulation of cell fate and patterning in the developing mammalian cochlea. Curr. Opin. Otolaryngol. Head Neck Surg. 17: 381–387.
Kelly MC and Chen P (2009) Development of form and function in the mammalian cochlea. Curr. Opin. Neurobiol. 19: 395–401.
Kiernan AE, Cordes R, Kopan R, Gossler A and Gridley T (2005a) The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development 132: 4353–4362.
Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP and Cheah KS (2005b) Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434: 1031–1035.
Kiernan AE, Xu J and Gridley T (2006) The Notch Ligand JAG1 Is Required for Sensory Progenitor Development in the Mammalian Inner Ear. PLoS Genet. 2: e4.
Ladher RK, Wright TJ, Moon AM, Mansour SL and Schoenwolf GC (2005) FGF8 initiates inner ear induction in chick and mouse. Genes Dev. 19: 603–613.
Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T and Kelley MW (1999) Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat. Genet. 21: 289–292.
Lanford PJ, Shailam R, Norton CR, Gridley T and Kelley MW (2000) Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. J. Assoc. Res. Otolaryngol. 1: 161–171.
Lee YS, Liu F and Segil N (2006) A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development 133: 2817–2826.
Liang JK, Bok J and Wu DK (2010) Distinct contributions from the hindbrain and mesenchyme to inner ear morphogenesis. Dev. Biol. 337: 324–334
Manoussaki D, Chadwick RS, Ketten DR, Arruda J, Dimitriadis EK and O’Malley JT (2008) The influence of cochlear shape on low-frequency hearing. Proc. Natl. Acad. Sci. USA 105: 6162–6166.
McKenzie E, Krupin A and Kelley MW (2004) Cellular growth and rearrangement during the development of the mammalian organ of Corti. Dev. Dyn. 229: 802–812.
Minowa O, Ikeda K, Sugitani Y, Oshima T, Nakai S, Katori Y, Suzuki M, Furukawa M, Kawase T, Zheng Y, et al. (1999) Altered cochlear fibrocytes in a mouse model of DFN3 nonsyndromic deafness. Science 285: 1408–1411.
Morsli H, Choo D, Ryan A, Johnson R and Wu DK (1998) Development of the mouse inner ear and origin of its sensory organs. J. Neurosci. 18: 3327–3335.
Ohyama T, Groves AK and Martin K (2007) The first steps towards hearing: mechanisms of otic placode induction. Int. J. Dev. Biol. 51: 463–472.
Ohyama T, Mohamed OA, Taketo MM, Dufort D and Groves AK (2006) Wnt signals mediate a fate decision between otic placode and epidermis. Development 133: 865–875.
Phippard D, Heydemann A, Lechner M, Lu L, Lee D, Kyin T and Crenshaw EB, 3rd (1998) Changes in the subcellular localization of the Brn4 gene product precede mesenchymal remodeling of the otic capsule. Hear. Res. 120: 77–85.
Pirvola U, Ylikoski J, Trokovic R, Hebert JM, McConnell SK and Partanen J (2002) FGFR1 is required for the development of the auditory sensory epithelium. Neuron 35: 671–680.
Powles N, Babbs C, Ficker M, Schimmang T and Maconochie M (2004) Identification and analysis of genes from the mouse otic vesicle and their association with developmental subprocesses through in situ hybridization. Dev. Biol. 268: 24–38.
Puligilla C, Feng F, Ishikawa K, Bertuzzi S, Dabdoub A, Griffith AJ, Fritzsch B and Kelley MW (2007) Disruption of fibroblast growth factor receptor 3 signaling results in defects in cellular differentiation, neuronal patterning, and hearing impairment. Dev. Dyn. 236: 1905–1917.
Raft S, Nowotschin S, Liao J and Morrow BE (2004) Suppression of neural fate and control of inner ear morphogenesis by Tbx1. Development 131: 1801–1812.
Riccomagno MM, Martinu L, Mulheisen M, Wu DK and Epstein DJ (2002) Specification of the mammalian cochlea is dependent on Sonic hedgehog. Genes Dev. 16: 2365–2378.
Riccomagno MM, Takada S and Epstein DJ (2005) Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 19: 1612–1623.
Schimmang T (2007) Expression and functions of FGF ligands during early otic development. Int. J. Dev. Biol. 51: 473–481.
Schlosser G (2006) Induction and specification of cranial placodes. Dev. Biol. 294: 303–351.
Shim K, Minowada G, Coling DE and Martin GR (2005) Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev. Cell 8: 553–564.
Spicer SS and Schulte BA (1991) Differentiation of inner ear fibrocytes according to their ion transport related activity. Hear. Res. 56: 53–64.
Steel KP and Smith RJ (1992) Normal hearing in Splotch (Sp/+), the mouse homologue of Waardenburg syndrome type 1. Nat. Genet. 2: 75–79.
Trowe MO, Maier H, Schweizer M and Kispert A (2008) Deafness in mice lacking the T-box transcription factor Tbx18 in otic fibrocytes. Development 135: 1725–1734.
Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, et al. (2005) Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat. Genet. 37: 980–985.
Wright TJ and Mansour SL (2003) Fgf3 and Fgf10 are required for mouse otic placode induction. Development 130: 3379–3390.
Zhang N, Martin GV, Kelley MW and Gridley T (2000) A mutation in the Lunatic fringe gene suppresses the effects of a Jagged2 mutation on inner hair cell development in the cochlea. Curr. Biol. 10: 659–662.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bok, J. Building the mammalian cochlea — an overview. Genes Genom 32, 1–7 (2010). https://doi.org/10.1007/s13258-010-0001-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13258-010-0001-1