Abstract
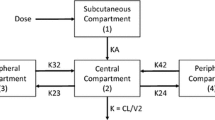
A randomized, double-blind, placebo-controlled, cross-over, dose-escalating, single-center study was conducted to evaluate the safety, tolerability, and pharmacokinetic (PK) profile of multiple once-daily (qd) subcutaneous (sc) doses of pasireotide in healthy male subjects. Subjects received pasireotide 50, 200, or 600 μg sc qd for 14 days and placebo in separate sequences. Thirty-three subjects were randomized. The most frequently reported drug-related adverse events were injection–site reactions (n = 18), diarrhea (n = 14) and nausea (n = 10), which were mostly mild or moderate in intensity. Pasireotide 600 μg sc was associated with pre- and post-prandial elevations in glucose levels relative to placebo; however, this effect was less pronounced on day 14 compared with day 1. PK steady state appeared to be achieved after 3 days of dosing and PK exposures had a moderate accumulation of 20–40 % across doses. Pasireotide demonstrated fast absorption (T max,ss: 0.25–0.5 h), low clearance (CL/F ss: 8.10–9.03 L/h), long effective half-life (T ½,eff: ~12 h, on average between 9.7 and 13.1 h for 50, 200, and 600 μg sc qd), and large volume of distribution (V z/F ss: 251–1,091 L) at steady state. Dose proportionality was confirmed for C max,ss; other PK parameters (C max, AUC0–24 h and AUCtau) were approximately dose proportional. Growth hormone inhibition was observed with pasireotide 200 and 600 μg sc qd. Gallbladder volume increased post-prandially with pasireotide 200 and 600 μg sc qd, which appeared to correlate with reduced levels of cholecystokinin at these doses. Pasireotide was generally well tolerated up to the tested dose of 600 μg qd, with a linear and time-independent PK profile after sc qd dosing in healthy subjects.




Similar content being viewed by others
References
U. Kumar, Cross-talk and modulation of signaling between somatostatin and growth factor receptors. Endocrine 40, 168–180 (2011)
A. Ben-Shlomo, H. Schmid, K. Wawrowsky et al., Differential ligand-mediated pituitary somatostatin receptor subtype signaling: implications for corticotroph tumor therapy. J. Clin. Endocrinol. Metab. 94, 4342–4350 (2009)
C. Bruns, I. Lewis, U. Briner, G. Meno-Tetang, G. Weckbecker, SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 146, 707–716 (2002)
H.A. Schmid, Pasireotide (SOM230): development, mechanism of action and potential applications. Mol. Cell. Endocrinol. 286, 69–74 (2008)
J. Van Der Hoek, W.W. de Herder, R.A. Feelders et al., A single-dose comparison of the acute effects between the new somatostatin analog SOM230 and octreotide in acromegalic patients. J. Clin. Endocrinol. Metab. 89, 638–645 (2004)
I. Donangelo, S. Melmed, Treatment of acromegaly: future. Endocrine 28, 123–128 (2005)
L. Kvols, J.E. Glusman, E.A. Hahn et al., The effects of pasireotide (SOM230) on health-related quality of life in patients with metastatic carcinoid tumors refractory or resistant to octreotide LAR. J. Clin. Oncol. 25(18S), abst 4558 (2007)
L. Kvols, B. Wiedenmann, K. Oberg et al., Safety and efficacy of pasireotide (SOM230) in patients with metastatic carcinoid tumors refractory or resistant to octreotide LAR: results of a Phase II study. ASCO Gastrointestinal Cancers Symposium, San Francisco, 26–28 January 2006, abst 171
K.I. Alexandraki, G. Kaltsas, Gastroenteropancreatic neuroendocrine tumors: new insights in the diagnosis and therapy. Endocrine 41, 40–52 (2012)
L.J. Hofland, S.W. Lamberts, The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr. Rev. 24, 28–47 (2003)
L.J. Hofland, J. Van Der Hoek, R. Feelders et al., The multi-ligand somatostatin analogue SOM230 inhibits ACTH secretion by cultured human corticotroph adenomas via somatostatin receptor type 5. Eur. J. Endocrinol. 152, 645–654 (2005)
D.L. Batista, X. Zhang, R. Gejman et al., The effects of SOM230 on cell proliferation and adrenocorticotropin secretion in human corticotroph pituitary adenomas. J. Clin. Endocrinol. Metab. 91, 4482–4488 (2006)
Y. Horsmans, K. Hu, M. Ruffin et al., Effect of hepatic impairment on the pharmacokinetics of pasireotide (SOM230): results from a multicenter Phase I study. J. Clin. Pharmacol. 52, 552–558 (2012)
P. Ma, Y. Wang, J. Van Der Hoek et al., Pharmacokinetic-pharmacodynamic comparison of a novel multiligand somatostatin analog, SOM230, with octreotide in patients with acromegaly. Clin. Pharmacol. Ther. 78, 69–80 (2005)
S. Petersenn, N. Unger, K. Hu et al., Pasireotide (SOM230), a novel multi-receptor-targeted somatostatin analogue, is well tolerated when administered as a continuous 7-day subcutaneous infusion in healthy male volunteers. J. Clin. Pharmacol. doi:10.1177/0091270011408727, [Epub ahead of print] (2011)
S. Petersenn, K. Hu, M. Maldonado et al., Tolerability and dose-proportional pharmacokinetics of pasireotide administered as a single dose or two divided doses in healthy male volunteers: a single-center, open-label, ascending-dose study. Clin. Ther. 34, 677–688 (2012)
M. Shenouda, M. Maldonado, Y. Wang et al., An open-label, dose-escalation study of once- and twice-daily pasireotide in healthy volunteers: safety, tolerability and effects on glucose, insulin and glucagon levels. Am. J. Ther. (2012 in press)
M. Boscaro, W.H. Ludlam, B. Atkinson et al., Treatment of pituitary dependent Cushing’s disease with the multi-receptor ligand somatostatin analog pasireotide (SOM230): a multicenter, Phase II trial. J. Clin. Endocrinol. Metab. 94, 115–122 (2009)
M. Boscaro, Y. Zhang, K. Sen et al., Long-term treatment of Cushing’s disease with pasireotide (SOM230): results from a Phase II extension study. Endocr. Rev. 31(3 Suppl 1), S333, abst P1-274 (2010)
M. Bronstein, F. Gu, C.-C. Shen et al. A randomized, blinded, multicenter, Phase III study to assess the efficacy and safety of pasireotide LAR versus octreotide LAR in patients with active acromegaly. 14th European NeuroEndocrine Association Congress, Liege, 22–25 September 2010, abst OC-6.1
A. Colao, J. Fleck, S. Pain et al., Pasireotide LAR versus octreotide LAR or lanreotide autogel in patients with inadequately controlled acromegaly: a Phase III, multicenter, randomized, parallel-group study. 14th European Neuroendocrine Association Congress, Liege, 22–25 September 2010, abst OC-6.2
A. Colao, S. Petersenn, J. Newell-Price et al., A 12-month Phase 3 study of pasireotide in Cushing’s disease. N. Engl. J. Med. 366, 914–924 (2012)
L. Kvols, K. Oberg, T.M. O’Dorisio et al., Efficacy, safety and pharmacokinetic results from a Phase II study of pasireotide (SOM230) in the treatment of patients with metastatic NETs refractory or resistant to octreotide LAR. Neuroendocrinology 92(1), abst C57 (2010)
S. Petersenn, J. Schopohl, A. Barkan et al., Pasireotide (SOM230) demonstrates efficacy and safety in patients with acromegaly: a randomized, multicenter, Phase II trial. J. Clin. Endocrinol. Metab. 95, 2781–2789 (2010)
H. Dietrich, K. Hu, M. Ruffin et al., Safety, tolerability and pharmacokinetics of a single dose of pasireotide long-acting release (LAR) in healthy volunteers: a single-center Phase I study. Eur. J. Endocrinol. doi:10.1530/EJE-11-0773, [Epub ahead of print] (2012)
Metropolitan Life Insurance Company, Metropolitan height and weight tables. Stat. Bull. 64, 2–9 (1983)
H. Boxenbaum, M. Battle, Effective half-life in clinical pharmacology. J. Clin. Pharmacol. 35, 763–766 (1995)
B.P. Smith, F.R. Vandenhende, K.A. DeSante et al., Confidence interval criteria for assessment of dose proportionality. Pharm. Res. 17, 1278–1283 (2000)
J. Hasskarl, M. Kaufmann, H.A. Schmid, Somatostatin receptors in non-neuroendocrine malignancies: the potential role of somatostatin analogs in solid tumors. Future Oncol. 7, 895–913 (2011)
G. Mazziotti, C. Gazzaruso, A. Giustina, Diabetes in Cushing syndrome: basic and clinical aspects. Trends Endocrinol. Metab. 22, 499–506 (2011)
R.R. Henry, S. Mudaliar, K. Wetli-Hermosillo et al., Mechanism and management of hyperglycemia associated with pasireotide: results from studies in healthy volunteers. Endocr. Rev. 32(03), abst P3-274 (2011)
Acknowledgments
This study was funded by Novartis Pharma AG. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Helene Darmofal for medical editorial assistance with this manuscript.
Conflict of interest
CB has no conflicts of interest regarding the content of this article. KH, YW, EB, CD, YfW, and PM are employees of Novartis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beglinger, C., Hu, K., Wang, Y. et al. Multiple once-daily subcutaneous doses of pasireotide were well tolerated in healthy male volunteers: a randomized, double-blind, placebo-controlled, cross-over, Phase I study. Endocrine 42, 366–374 (2012). https://doi.org/10.1007/s12020-012-9668-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9668-1