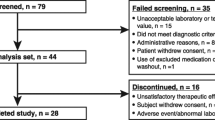
Abstract
Purpose
To assess the pharmacokinetics, pharmacodynamics, safety and tolerability of subcutaneous depot CAM4071, a novel, ready-to-use pasireotide formulation.
Methods
This was a phase 1, randomised, open-label study in healthy volunteers. After a single 600 µg dose of pasireotide immediate release (IR), participants were randomised to one of eight groups to receive either a CAM4071 upper thigh (5, 10, 20, 40 or 80 mg) or buttock (20 mg) injection or multiple pasireotide IR 900 µg upper thigh injections twice daily or a single pasireotide long-acting release (LAR) 60 mg intramuscular buttock injection.
Results
Ninety-four participants were randomised. For all CAM4071 doses, initial pasireotide release was relatively rapid compared to pasireotide LAR and sustained over the 2-month observation period, with a slow decay in plasma concentrations. CAM4071 maximum plasma concentrations increased slightly greater than dose proportionally; area under the curve extrapolated to infinity increased approximately dose proportionally. Relative bioavailability of pasireotide for different doses of CAM4071 versus pasireotide IR 600 μg ranged from 0.752 (90% confidence interval [CI]: 0.58, 0.98) to 1.68 (1.32, 2.14), and versus pasireotide LAR: 0.517 (0.37, 0.72) to 1.15 (0.84, 1.58). CAM4071 doses >5 mg exhibited rapid initial reductions of insulin-like growth factor 1 (IGF-1) compared to pasireotide LAR. Maximum IGF-1 inhibition was greatest for CAM4071 80 mg. CAM4071 injections ≤40 mg were well tolerated and comparable with currently available pasireotide formulations.
Conclusion
CAM4071 provided long-acting release of pasireotide over at least one month, with high bioavailability and onset and duration of IGF-1 suppression similar to pasireotide LAR.
Trial registration
EudraCT: 2014-003783-20.



Similar content being viewed by others
References
A. Colao, L.F. Grasso, A. Giustina, S. Melmed, P. Chanson et al. Acromegaly. Nat. Rev. Dis. Primers 5, 20 (2019).
I. Shimon, Somatostatin receptors in pituitary and development of somatostatin receptor subtype-selective analogs. Endocrine 20, 265–269 (2003).
T. Eigler, A. Ben-Shlomo, C. Zhou, R. Khalafi, S.-G. Ren et al. Constitutive somatostatin receptor subtype-3 signaling suppresses growth hormone synthesis. Mol. Endocrinol. 28, 554–564 (2014).
H.A. Schmid, Pasireotide (SOM230): development, mechanism of action and potential applications. Mol. Cell. Endocrinol. 286, 69–74 (2008).
A. Akirov, A. Gorshtein, I. Dotan, N.S. Khazen, Y. Pauker et al. Long-term safety and efficacy of long-acting pasireotide in acromegaly. Endocrine 74, 396–403 (2021).
M. Fleseriu, Clinical efficacy and safety results for dose escalation of somatostatin receptor ligands in patients with acromegaly: a literature review. Pituitary 14, 184–193 (2011).
A. Colao, M. Bronstein, P. Freda, F. Gu, C.-C. Shen, et al. Pasireotide versus octreotide in acromegaly: a head-to-head superiority study. J. Clin. Endocrinol. Metabol. 99, 791–799 (2014).
S. Petersenn, J. Schopohl, A. Barkan, P. Mohideen, A. Colao, et al. Pasireotide (SOM230) demonstrates efficacy and safety in patients with acromegaly: a randomized, multicenter, phase II trial. J. Clin. Endocrinol. Metab. 95, 2781–2789 (2010).
S. Fattah, D.J. Brayden, Progress in the formulation and delivery of somatostatin analogs for acromegaly. Ther. Deliv. 8, 867–878 (2017).
M. Pavel, F. Borson-Chazot, A. Cailleux, D. Hörsch, H. Lahner, et al. Octreotide SC depot in patients with acromegaly and functioning neuroendocrine tumors: a phase 2, multicenter study. Cancer Chemother. Pharmacol. 83, 375–385 (2019).
R.J. Comi, Octreotide: a 15-year update. Endocrinologist 16, 86–90 (2006).
C. Bruns, I. Lewis, U. Briner, G. Meno-Tetang, G. Weckbecker, SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur. J. Endocrinol. 146, 707–716 (2002).
M.R. Gadelha, M.D. Bronstein, T. Brue, M. Coculescu, M. Fleseriu, et al. Pasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol 2, 875–884 (2014).
A. Colao, S. Petersenn, J. Newell-Price, J.W. Findling, F. Gu, et al. A 12-month phase 3 study of pasireotide in Cushing’s disease. N. Engl. J. Med. 366, 914–924 (2012).
D. Cuevas-Ramos, M. Fleseriu, Pasireotide: a novel treatment for patients with acromegaly. Drug Des. Dev. Ther. 10, 227–239 (2016).
M. Fleseriu, B.M. Biller, P.U. Freda, M.R. Gadelha, A. Giustina, et al. A Pituitary Society update to acromegaly management guidelines. Pituitary 24, 1–13 (2021).
FDA: SIGNIFOR LAR® highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203255s004lbl.pdf (2018). Accessed 7 Jun 2023.
EMA: SIGNIFOR® LAR Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/signifor-epar-product-information_en.pdf (2017). Accessed 07 Jun 2023.
PMDA: Signifor LAR. Report on the Deliberation Results. https://www.pmda.go.jp/files/000234111.pdf (2018). Accessed 28 Jun 2023.
J.M. Silverstein, Hyperglycemia induced by pasireotide in patients with Cushing’s disease or acromegaly. Pituitary 19, 536–543 (2016).
K. McKeage, Pasireotide in acromegaly: a review. Drugs 75, 1039–1048 (2015).
R.R. Henry, T.P. Ciaraldi, D. Armstrong, P. Burke, M. Ligueros-Saylan et al. Hyperglycemia associated with pasireotide: results from a mechanistic study in healthy volunteers. J. Clin. Endocrinol. Metab. 98, 3446–3453 (2013).
S. Chatterjee, K. Khunti, M.J. Davies, Optimizing management of glycaemia. Best Pract. Res. Clin. Endocrinol. Metab. 30, 397–411 (2016).
F. Tiberg, J. Roberts, C. Cervin, M. Johnsson, S. Sarp et al. Octreotide sc depot provides sustained octreotide bioavailability and similar IGF‐1 suppression to octreotide LAR in healthy volunteers. Br. J. Clin. Pharmacol. 80, 460–472 (2015).
F. Tiberg, M. Johnsson, M. Jankunec, J. Barauskas, Phase behavior, functions, and medical applications of soy phosphatidylcholine and diglyceride lipid compositions. Chem. Lett. 41, 1090–1092 (2012).
F. Tiberg, M. Johnsson, Drug delivery applications of non-lamellar liquid crystalline phases and nanoparticles. J. Drug Deliv. Sci. and Technol. 21, 101–109 (2011).
Novartis: CSOM230C1202 Protocol Summary. https://classic.clinicaltrials.gov/ProvidedDocs/46/NCT01673646/Prot_001.pdf (2014). Accessed 28 Jun 2023.
FDA: SIGNIFOR (pasireotide) injection. Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/200677s003lbl.pdf (2012). Accessed 04 Jul 2023.
H. Dietrich, K. Hu, M. Ruffin, D. Song, E. Bouillaud et al. Safety, tolerability, and pharmacokinetics of a single dose of pasireotide long-acting release in healthy volunteers: a single-center Phase I study. Eur J. Endocrinol. 166, 821–828 (2012).
X. Chen, G. Shen, J. Jiang, H. Liu, K. Hu et al. Pharmacokinetics and safety of subcutaneous pasireotide and intramuscular pasireotide long-acting release in Chinese male healthy volunteers: a phase I, single-center, open-label, randomized study. Clin. Ther. 36, 1196–1210 (2014).
S. Petersenn, J. Bollerslev, A.M. Arafat, J. Schopohl, O. Serri et al. Pharmacokinetics, pharmacodynamics, and safety of pasireotide LAR in patients with acromegaly: a randomized, multicenter, open‐label, phase I study. J. Clin. Pharmacol. 54, 1308–1317 (2014).
J.M. Fernandez, S. Madsen, J.M. Krase, V.Y. Shi, Classification and mitigation of negative injection experiences with biologic medications. Dermatol. Ther. 33, e13240 (2020).
A. Frid, J.-E. Hardebo, The thigh may not be suitable as an injection site for patients self-injecting sumatriptan. Neurology 49, 559–561 (1997).
M.R. Gadelha, F. Gu, M.D. Bronstein, T.C. Brue, M. Fleseriu et al. Risk factors and management of pasireotide-associated hyperglycemia in acromegaly. Endocrine Connections 9, 1178 (2020).
Acknowledgements
The authors thank the volunteers, the investigators and their teams who took part in this study and the clinical trial that the data were based on. The authors also acknowledge Harry Smith, MSc, Sunandan Dhar, MTech, and James Evry, MSc, Costello Medical, UK, for medical writing and editorial assistance based on the authors’ input and direction. This study was sponsored by Camurus AB.
Funding
This analysis was sponsored by Camurus AB. Support for third-party writing assistance for this article, provided by Harry Smith, MSc, Sunandan Dhar, MTech, and James Evry, MSc, Costello Medical, UK, was funded by Camurus AB in accordance with Good Publication Practice 2022 guidelines (GPP 2022 [ismpp.org]).
Author information
Authors and Affiliations
Contributions
F.T., A.M.P. and M.J. made substantial contributions to the study conception and design. F.T., A.M.P., M.J. and A.H. made substantial contributions to analysis and interpretation of the data. All authors draughted the article or revised it critically for important intellectual content. All authors approved the final version of the article to be published.
Corresponding author
Ethics declarations
Conflict of interest
F.T., A.M.P., M.J. and A.H. are current employees of Camurus AB.
Ethics approval
This trial was conducted in accordance with the ethical principles that have their origins in the Declaration of Helsinki and in compliance with the approved protocol, Guidelines of the International Council for Harmonisation on Good Clinical Practice and applicable regulatory requirements. The study was approved by an independent ethics committee (Ethics Committee of the State of Berlin), approval number 14/0518 – EK 13.
Consent to participate
Written informed consent was obtained from all participants included in the study.
Consent to publish
All the results presented in this article are in aggregate form, and no personally identifiable information was used for this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Johnsson, M., Pedroncelli, A.M., Hansson, A. et al. Pharmacokinetics and pharmacodynamics of a pasireotide subcutaneous depot (CAM4071) and comparison with immediate and long-acting release pasireotide. Endocrine (2024). https://doi.org/10.1007/s12020-024-03741-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12020-024-03741-3