Abstract
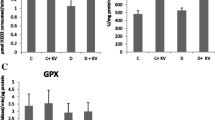
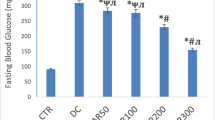
Effects of vitamin E and selenium supplementation on aldehyde oxidase (AO) and xanthine oxidase (XO) activities and antioxidant status in liver, kidney, and heart of streptozotocin (STZ)-induced diabetic rats were examined. AO and XO activities increased significantly after induction of diabetes in rats. Following oral vitamin E (300 mg/kg) and sodium selenite (0.5 mg/kg) intake once a day for 4 weeks, XO activity decreased significantly. AO activity decreased significantly in liver, but remained unchanged in kidney and heart of vitamin E- and selenium-treated rats compared to the diabetic rats. Total antioxidants status, paraoxonase-1 (PON1) and erythrocyte superoxide dismutase activities significantly decreased in the diabetic rats compared to the controls, while a higher fasting plasma glucose level was observed in the diabetic animals. The glutathione peroxidase activity remained statistically unchanged. Malondialdehyde and oxidized low-density lipoprotein levels were higher in the diabetic animals; however, these values were significantly reduced following vitamin E and selenium supplementation. In summary, both AO and XO activities increase in STZ-induced diabetic rats, and vitamin E and selenium supplementation can reduce these activities. The results also indicate that administration of vitamin E and selenium has hypolipidemic, hypoglycemic, and antioxidative effects. It decreases tissue damages in diabetic rats, too.



Similar content being viewed by others
References
King H, Aubert RE, Herman WH (1998) Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 21:1414–1431
Strain JJ (1991) Disturbances of micronutrient and antioxidant status in diabetes. Proceedings of the Nutrition Society 50:591–604
Ramakrishna V, Jailkhani R (2008) Oxidative stress in non-insulin-dependent diabetes mellitus (NIDDM) patients. Acta Diabetol 45:41–46
Desco MC, Asensi M, Marquez R et al (2002) Xanthine oxidase is involved in free radical production in type 1 diabetes: Protection by allopurinol. Diabetes 51:1118–1124
Hunt JV, Dean RT, Wolff SP (1988) Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J 256:205–212
Jain SK, McVie R, Jaramillo JJ, Chen Y (1998) Hyperketonemia (acetoacetate) increases the oxidizability of LDL + VLDL in Type-I diabetic patients. Free Radic Biol Med 24:175–181
Inkster ME, Cotter MA, Cameron NE (2007) Treatment with the xanthine oxidase inhibitor, allopurinol, improves nerve and vascular function in diabetic rats. Eur J Pharmacol 561:63–71
Kundu TK, Hille R, Velayutham M, Zweier JL (2007) Characterization of superoxide production from aldehyde oxidase: an important source of oxidants in biological tissues. Arch Biochem Biophys 460:113–121
Higgins P, Ferguson LD, Walters MR (2011) Xanthine oxidase inhibition for the treatment of stroke disease: a novel therapeutic approach. Expert Rev Cardiovasc Ther 9:399–401
Higgins P, Dawson J, Walters M (2009) The potential for xanthine oxidase inhibition in the prevention and treatment of cardiovascular and cerebrovascular disease. Cardiovasc Psychiatry Neurol 2009:282059.
Hanachi N, Charef N, Baghiani A et al (2009) Comparison of xanthine oxidase levels in synovial fluid from patients with rheumatoid arthritis and other joint inflammations. Saudi Med J 30:1422–1425
Lee BE, Toledo AH, Anaya-Prado R, Roach RR, Toledo-Pereyra LH (2009) Allopurinol, xanthine oxidase, and cardiac ischemia. J Investig Med 57:902–909
Matsumoto S, Koshiishi I, Inoguchi T, Nawata H, Utsumi H (2003) Confirmation of superoxide generation via xanthine oxidase in streptozotocin-induced diabetic mice. Free Radic Res 37:767–772
Turner NA, Doyle WA, Ventom AM, Bray RC (1995) Properties of rabbit liver aldehyde oxidase and the relationship of the enzyme to xanthine oxidase and dehydrogenase. Eur J Biochem 232:646–657
Rashidi MR, Nazemiyeh H (2010) Inhibitory effects of flavonoids on molybdenum hydroxylases activity. Expert Opin Drug Metab Toxicol 6:133–152
Gupta S, Sharma TK, Kaushik GG, Shekhawat VP (2011) Vitamin E supplementation may ameliorate oxidative stress in type 1 diabetes mellitus patients. Clin Lab 57:379–386
Pazdro R, Burgess JR (2010) The role of vitamin E and oxidative stress in diabetes complications. Mech Ageing Dev 131:276–286
Beales PE, Williams AJ, Albertini MC, Pozzilli P (1994) Vitamin E delays diabetes onset in the non-obese diabetic mouse. Horm Metab Res 26:450–452
Faure P (2003) Protective effects of antioxidant micronutrients (vitamin E, zinc and selenium) in type 2 diabetes mellitus. Clin Chem Lab Med 41:995–998
Ruíz C, Alegría A, Barberá R, Farré R, Lagarda J (1998) Selenium, zinc and copper in plasma of patients with type 1 diabetes mellitus in different metabolic control states. J Trace Elem Med Biol 12:91–95
Navarro-Alarcón M, López-G de la Serrana H, Pérez-Valero V, López-Martínez C (1999) Serum and urine selenium concentrations as indicators of body status in patients with diabetes mellitus. Sci Total Environ 228:79–85
Marcason W (2008) What is the latest research on the connection between selenium and diabetes? J Am Diet Assoc 108:188
Sotiropoulos A, Papadodima SA, Papazafiropoulou AK, Ioannidis A, Kokkinari A, Apostolou O, Spiliopoulou CA, Athanaselis S (2011) Serum selenium levels do not differ in type 2 diabetic subjects with and without coronary artery disease. BMC Res Notes 4:270
Bleys J, Navas-Acien A, Guallar E (2007) Serum selenium and diabetes in U.S. adults. Diabetes Care 30:829–834
Steinbrenner H, Speckmann B, Pinto A, Sies H (2011) High selenium intake and increased diabetes risk: experimental evidence for interplay between selenium and carbohydrate metabolism. J Clin Biochem Nutr 48:40–45
Fernandes AAH, Novelli ELB, Okoshi K et al (2010) Influence of rutin treatment on biochemical alterations in experimental diabetes. Biomedicine and Pharmacotherapy 64:214–219
Ramesh B, Pugalendi KV (2006) Antioxidant role of Umbelliferone in STZ-diabetic rats. Life Sciences 79:306–310
Pirouzpanah S, Hanaee J, Razavieh SV, Rashidi MR (2009) Inhibitory effects of flavonoids on aldehyde oxidase activity. Enz Inhibit Med Chem 24:14–21
Rashidi MR, Amini K, Khani MY, Faridi A, Hanaee J, Sorouraddin MS (2011) A highly sensitive HPLC-florescence method to study aldehyde oxidase activity. JAOAC Int 94:550–554
Prajda N, Weber G (1975) Malignant transformation linked imbalance: decreased xanthine oxidase activity in hepatomas. FEBS Letters 59:245–249
Burstein M, Scholnick HR, Morfin R (1970) Rapid method for isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res 11(6):583–595
Chait A, Brazg RL, Tribble DL, Krauss RM (1993) Susceptibility of small, dense, low-density lipoproteins to oxidative modification in subjects with the atherogenic lipoprotein phenotype, pattern B. Am J Med 94(4):350–356
Drabkin DL (1965) The molecular weight of haemoglobin, its iron and nitrogen content and optical properties—their relevance in the problem of a reference standard for haemoglobin measurement. Bibl Haematol 21:33–42
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Ogura R, Ueta H, Sugiyama M, Hidaka T (1990) Distribution of superoxide dismutase activity in the epidermis: measurement with electron spin resonance spin trapping. J Invest Dermatol 94:227–229
Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A (1993) A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond) 84(4):407–412
Gallou G, Ruelland A, Legras B, Maugendre D, Allannic H, Cloarec L (1993) Plasma malondialdehyde in type 1 and type 2 diabetic patients. Clinica Chimica Acta 214:227–234
Jafarnejad A, Bathaie SZ, Nakhjavani M, Hassan MZ (2008) Effect of spermine on lipid profile and HDL functionality in the streptozotocin-induced diabetic rat model. Life Sciences 82(5–6):301–307
Sathishsekar D, Subramanian S (2005) Antioxidant properties of Momordica charantia (bitter gourd) seeds on Streptozotocin induced diabetic rats. Asia Pacific Journal of Clinical Nutrition 14:153–158
Johansen JS, Harris AK, Rychly DJ, Ergul A (2005) Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol 4:5
Akirav EM, Chan O, Inouye K, Riddell MC, Matthews SG, Vranic M (2004) Partial leptin restoration increases hypothalamic-pituitary-adrenal activity while diminishing weight loss and hyperphagia in streptozotocin diabetic rats. Metabolism 53:1558–1564
Ihara Y, Toyokuni S, Uchida K et al (1999) Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes 48:927–932
Chen V, Ianuzzo CD (1982) Dosage effect of streptozotocin on rat tissue enzyme activities and glycogen concentration. Can J Physiol Pharmacol 60:1251–1256
Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50:537–546
Xue S, Chen X, Lu J, Jin L (2009) Protective effect of sulfated Achyranthes bidentata polysaccharides on streptozotocin-induced oxidative stress in rats. Carbohydrate Polymers 75:415–419
Tiedge M, Lortz S, Drinkgern J, Lenzen S (1997) Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 46:1733–1742
Aliciguzel Y, Ozen I, Aslan M, Karayalcin U (2003) Activities of xanthine oxidoreductase and antioxidant enzymes in different tissues of diabetic rats. J Lab Clin Med 142:172–177
Dedon PC, Plastaras JP, Rouzer CA, Marnett LJ (1998) Indirect mutagenesis by oxidative DNA damage: formation of the pyrimidopurinone adduct of deoxyguanosine by base propenal. Proc Natl Acad Sci USA 95:11113–11116
Garattini E, Fratelli M, Terao M (2008) Mammalian aldehyde oxidases: genetics, evolution and biochemistry. Cell Mol Life Sci 65:1019–1048
Krenitsky TA, Spector T, Hall WW (1986) Xanthine oxidase from human liver: purification and characterization. Arch Biochem Biophys 247:108–119
Rodrigues AD (1994) Comparison of levels of aldehyde oxidase with cytochrome P450 activities in human liver in vitro. Biochem Pharmacol 48:197–200
Acknowledgements
This work was supported by a grant from Nutrition Research Center, Medical Faculty and Students’ Research Committee, Tabriz University of Medical Sciences. This is a report of a database from thesis entitled “Effect of Vitamin E and Selenium on Lipid Peroxidation in Diabetic Rats”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghaffari, T., Nouri, M., Saei, A.A. et al. Aldehyde and Xanthine Oxidase Activities in Tissues of Streptozotocin-Induced Diabetic Rats: Effects of Vitamin E and Selenium Supplementation. Biol Trace Elem Res 147, 217–225 (2012). https://doi.org/10.1007/s12011-011-9291-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9291-7