Abstract
Compressive osseointegration technology, which provides immediate, mechanically compliant endoprosthetic fixation, has been adapted for massive proximal tibial reconstructions in an attempt to avoid aseptic failure encountered with conventional stems. A retrospective review of 16 patients with resected tumors was undertaken to determine whether compressive osseointegration can provide durable anchorage of tibial implants. Medical records, radiographs, and clinical examinations were reviewed to assess surgical, local disease control, and prosthetic outcomes. The average age was 18 years (range, 12–42 years). Diagnoses included osteosarcoma (12), Ewing sarcoma (two), chondrosarcoma (one), and undifferentiated sarcoma (one). Minimum followup was 2 years (mean, 4.5 years; range, 2–10.3 years); no patient was lost to followup. There were no local recurrences. Four patients developed metastatic disease; one patient died of his primary tumor, and another died from a chemotherapy-related malignancy. Complications included one early deep infection that ultimately resulted in prosthetic loosening and the need for an above-knee amputation. There were two late deep infections; prosthetic retention was achieved with débridement and antibiotics. One patient developed aseptic loosening and underwent revision; the other 15 implants provided stable osseointegration at last followup. Compressive osseointegration technology can thus achieve acceptable short-term endoprosthetic fixation results and may reduce the risk of aseptic loosening reported with conventional tibial stems.
Level of Evidence: Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Similar content being viewed by others
Introduction
A good deal has been reported about the outcomes of massive distal femoral endoprosthetic reconstructions after tumor resection. Recent studies have described distal femoral endoprosthetic survivorship at 10 years to range upward of 80% [6, 31, 39]. Less is known about proximal tibial oncologic reconstructive outcomes for a number of reasons. First, although the incidence of proximal tibial tumors ranks next to distal femoral lesions, the latter remain roughly twice as common [48]. Second, amputation is more often necessary for proximal tibial neoplasms because of neurovascular involvement. Third, the comparatively greater challenges posed by the reestablishment of extensor mechanism function after proximal tibial resection have led to the use of a variety of tibial reconstructive methods, including arthrodesis [12], allografts [10, 14, 43], and alloprostheses [5, 17, 51] with relatively fewer endoprosthetic reconstructions. Furthermore, although intermediate-term (ie, 5–10 years) prosthetic survivorship of a variety of distal femoral implants is reasonably predictable and acceptable [6, 33, 41, 45], tibial endoprosthetic reconstruction is more challenging principally because of the biomechanical demands placed on tibial stems. Two- to 5-year failure rates resulting from aseptic loosening have ranged from 3% to 46% in a variety of series [19, 22, 24, 26, 29, 34, 35].
Compressive osseointegration technology was developed in an attempt to provide secure, long-term anchorage of oncologic endoprostheses by using a spring-loaded device to achieve compliant prestress fixation, thus avoiding complications of stress shielding and particle-induced osteolysis [8, 15, 27, 36]. Initial distal femoral radiographic results have confirmed progressive bone hypertrophy at the prosthetic interface [3] and early clinical comparisons with cemented stems [4] have demonstrated equivalent prosthetic survivorship at 2 years.
For primary tumor proximal tibial resections managed with compressive osseointegration reconstructions, the purposes of this study were to determine (1) the rates of local control and prosthetic survival; (2) the frequency and nature of surgical complications; (3) and the outcome of prosthetic revision.
Materials and Methods
I retrospectively reviewed 16 patients with resected malignancies reconstructed with a proximal tibial Compress® device (Biomet, Inc, Warsaw, IN) between April 1998 and September 2006. There were seven males and nine females with an average age of 18 years (range, 12–42 years). Diagnoses included osteosarcoma (12), Ewing’s sarcoma (two), chondrosarcoma (one), and undifferentiated sarcoma (one). Distal femoral Compress® devices, initially introduced in 1993, were granted US Food and Drug Administration clearance in December 2003. On a custom off-label basis, Compress® implants have been available for proximal tibial reconstructions since 1998. The minimum followup was 2 years (mean, 4.5 years; range, 2–10.3 years). No patient was lost to followup. Prior Institutional Review Board approval was obtained for this retrospective review.
Previously published methods of proximal tibial resection and endoprosthetic reconstruction were followed [7, 13, 16, 18, 23, 28, 30, 36, 50]. A sufficient amount of proximal tibia was removed to achieve negative surgical margins. Resection length averaged 17 cm (range, 13–24 cm); remaining distal tibial segments averaged 20 cm (range, 11–25 cm); percentage of tibia resected averaged 46% (range, 35%–65%). The tibial canal was reamed to allow placement of a 10-mm anchor plug and a centering sleeve of at least 12 mm diameter. Compression force (in pounds) was as follows: 400 (five), 600 (10), and 800 (one); two short custom spindles were used. Over time, we have empirically preferred 600-pound small, short hydroxyapatite spindles for tibial reconstructions. Twelve patients underwent extensor mechanism reinforcement and soft tissue coverage with a gastrocnemius flap.
Continuous passive motion of the knee was begun 48 hours after surgery or as soon as permitted by the plastic surgery staff. Quadriceps sets and straight leg raising exercises were begun 2 weeks after surgery, but progressive resistive exercises were not undertaken. In contrast to rehabilitation of patients with cemented stems, for which full weight can be borne immediately, weightbearing on the tibial Compress® implant was withheld for 3 months postoperatively, after which time weightbearing was advanced at a rate of 25% of body weight per week.
Followup visits for routine clinical and radiographic examinations generally occurred at 2, 6, and 12 weeks and at 3-month intervals thereafter. Medical records were reviewed to obtain demographic data, including age, gender, diagnosis, and treatment information. Operative reports were studied to record technical factors, including implant length relative to remaining distal tibial length, compression force, and spindle length. Major complications such as aseptic loosening, infection, local recurrence, periprosthetic fracture, need for further surgery, metastatic disease, and death were recorded. Device-related mechanical failure was defined as the need for revision secondary to aseptic loosening.
I assessed postoperative radiographs at 3- to 6-month intervals for indications of technical error (pin malposition or migration) and for evidence of device-related failure (lucency at the bone-prosthetic interface, endosteal erosion, loss of compression distance, gross loosening, and implant breakage).
Results
There were no local recurrences. Four patients had metastatic disease at last followup. Two deaths occurred; one patient died secondary to osteosarcoma, and one developed myelofibrosis.
Surgical complications included one early deep infection that ultimately resulted in prosthetic loosening and the need for an above-knee amputation. There were two late deep prosthetic infections; prosthetic retention was achieved with débridement and antibiotics. There were two nondisplaced tibial fractures, not associated with the hardware, which were treated nonoperatively. There were no prosthetic fractures or other forms of mechanical breakage. All patients were able to walk without an assistive device.
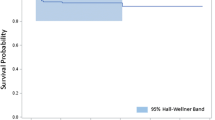
There was one device-related aseptic mechanical failure of the Compress® device (Fig. 1). At 3.2 years after the index procedure, revision to a slightly longer Compress® tibial replacement was successfully accomplished. All other implants demonstrated radiographic signs of stable osseointegration as evidenced by bone hypertrophy at the prosthetic interface and lack of stress shielding (Fig. 2). Design modifications over the study period, in terms of compression force or spindle type, did not correlate with any observable changes in the degree of osseointegration.
Discussion
The rationale of this study was to better understand the short-term outcomes of compressive osseointegration technology when used for reconstruction of massive defects after primary oncologic resection. The primary aims were the determination of prosthetic survival and management of revisions. The secondary aims were documentation of local control and surgical complications.
Limitations of this study include the small number of patients, the lack of control subjects, and the limited followup. The limited study size makes statistical analysis difficult, especially in terms of meaningful calculation of implant survival analysis. With average 4.5-year followup, the data in this study should be viewed as preliminary with results needing to be further developed to substantiate conclusions regarding prosthetic longevity. However, given the relative infrequency of primary oncologic proximal tibial reconstructions, this review of 16 patients is one of the larger series of uncemented devices and the only one that relates to compressive osseointegration technology. Except for the two patients who died, all of the patients reported continue to be examined regularly so the longer-term durability of the implant can be determined.
Effective reconstruction of massive defects after resection of proximal tibial neoplasms is challenging for several reasons. First, as compared with femoral presentations, the size of tibial tumors and the close proximity of surrounding neurovascular structures often render decision-making regarding limb salvage difficult. As compared with distal femoral tumors, achieving local control of tibial lesions more often necessitates amputation. The correspondingly fewer patients who do receive prosthetic reconstructions may still be at high risk for local recurrence. Although not directly a product of osseointegration technology, the finding that all patients in this study demonstrated local control is nonetheless reassuring in terms of validating a limb salvage approach in a carefully selected population.
A second difficulty of tibial prosthetic reconstruction is the considerable biomechanical stress placed on conventional tibial intramedullary stems, which has been associated with relatively high rates of mechanical failure resulting from prosthetic fracture and aseptic loosening. Determination of the influence of mechanical failure on rates of proximal tibial prosthetic survivorship, based on the extant literature, is difficult. Many studies combine outcomes of proximal tibial implants with those from other anatomic locations, including the distal and proximal femur [2, 5, 9, 20, 21, 26, 29, 31, 32, 38–40, 42, 44, 47, 49, 51]. When reported separately, proximal tibial results frequently detail all-cause failure combining the effects of infection, local recurrence, fracture, and other mechanical issues on prosthetic retention [26, 29, 31, 32, 47]. Some papers have few patients and/or short followup [1, 2, 9, 37, 52, 53], whereas some that describe experience extending over several decades combine the findings with several distinct proximal tibial implant designs and surgical techniques [5, 26, 29, 32, 39, 42, 47]. To the extent that rates of proximal tibial endoprosthetic mechanical failure can be determined from the literature over the past 20 years, most authors report figures of 10% to 30% failure at 5- to 10-year followup (Table 1). The most promising long-term results were reported by Myers et al, who described a 9% rate of aseptic loosening in 99 rotating hinge devices at 15 years of followup, although seven prosthetic fractures should be added to estimate the actual mechanical failure rate [34]. Subsequently, this group reported longer experience with a variety of proximal tibial implants, for which mechanical failure rates of 37.5% and 59.4% were reported at 10 and 20 years, respectively [25]. The addition of porous- or hydroxyapatite-coated collars at the bone-prosthetic interface seems to hold the promise of improving longevity of conventional cemented or uncemented stems [9, 34, 46]. This study of primary oncologic reconstructions was undertaken to specifically determine the intermediate-term mechanical failure rate of a single type of proximal tibial endoprosthesis that uses a novel means of achieving compressive osseointegration. At an average of 4.5 years followup, only one prosthetic failure resulting from mechanical loosening of the implant was noted. This result compares favorably with other types of proximal tibial implants reported in the literature. The Compress® device would thus seem to provide an effective means of providing stable fixation for massive proximal tibial reconstructions after primary oncologic resection. Initial results suggest Compress® technology may well serve to avoid the problems of prosthetic breakage, stress shielding, and particle-induced osteolytic loosening that are associated with conventional tibial stems.
A third problematic aspect of tibial reconstruction involves soft tissue coverage and the consequent risk of prosthetic infection. One patient without flap coverage in this series developed an infection that ultimately necessitated an amputation; the two other patients who developed a deep infection retained their implants, perhaps in part because they had received a flap as part of their index procedure. The utility of the gastrocnemius flap in decreasing the risk of tibial prosthetic infection and in improving extensor mechanism reconstitution serves to makes its use routine [7, 11].
A final challenge of tibial reconstruction is the frequent need to salvage short metadiaphyseal fragments remaining after tumor resection or revision. The “bone-sparing” nature of the Compress® device (as little as 43 mm of bone can be implanted) and the relative ease of revision after infection, fracture, or mechanical loosening (the device is readily removed and as little as 1 cm of additional bone needs to be resected at the time of reimplantation) are highlighted by the successful revision of the sole case of mechanical failure (Fig. 1) [36].
References
Abboud JA, Patel RV, Donthineni-Rao R, Lackman RD. Proximal tibial prosthetic replacement without the use of muscle flaps. Clin Orthop Relat Res. 2003;414:189–196.
Ahlmann ER, Menendez LR, Kermani C, Gotha H. Survivorship and clinical outcome of modular endoprosthetic reconstruction for neoplastic disease of the lower limb. J Bone Joint Surg Br. 2006;88:790–795.
Avedian RS, Goldsby RE, Kramer MJ, O’Donnell RJ. Effect of chemotherapy on initial compressive osseointegration of tumor endoprostheses. Clin Orthop Relat Res. 2007;459:48–53.
Bhangu AA, Kramer MJ, Grimer RJ, O’Donnell RJ. Early distal femoral endoprosthetic survival: cemented stems versus the Compress implant. Int Orthop. 2006;30:465–472.
Biau D, Faure F, Katashian S, Jeanrot C, Tomeno B, Anract P. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J Bone Joint Surg Am. 2006;88:1285–1293.
Bickels J, Wittig JC, Kollender Y, Henshaw RM, Kellar-Graney KL, Meller I, Malawer M. Distal femoral resection with endoprosthetic reconstruction: a long-term followup study. Clin Orthop Relat Res. 2002;400:225–235.
Bickels J, Wittig JC, Kollender Y, Neff RS, Kellar-Graney K, Meller I, Malawer MM. Reconstruction of the extensor mechanism after proximal tibia endoprosthetic replacement. J Arthroplasty. 2001;16:856–862.
Bini SA, Johnston JO, Martin DL. Compliant prestress fixation in tumor prostheses: interface retrieval data. Orthopedics. 2000;23:707–712.
Blunn GW, Briggs TWR, Cannon SR, Walker PS, Unwin PS, Culligan S, Cobb JP. Cementless fixation for primary segmental bone tumor endoprostheses. Clin Orthop Relat Res. 2000;372:223–230.
Brien EW, Terek RM, Healey JH, Lane JM. Allograft reconstruction after proximal tibial resection for bone tumors: an analysis of function and outcome comparing allograft and prosthetic reconstructions. Clin Orthop Relat Res. 1994;303:116–127.
Busfield BT, Huffman GR, Nahai F, Hoffman W, Ries MD. Extended medial gastrocnemius rotational flap for treatment of chronic knee extensor mechanism deficiency in patients with and without total knee arthroplasty. Clin Orthop Relat Res. 2004;428:190–197.
Campanacci M, Costa P. Total resection of distal femur or proximal tibia for bone tumours: autogenous bone grafts and arthrodesis in twenty-six cases. J Bone Joint Surg Br. 1979;61:455–463.
Cannon CP, Zeegen E, Eckardt JJ. Techniques in endoprosthetic reconstruction. Operative Techniques in Orthopaedics. 2005;14:225–235.
Clohisy DR, Mankin HJ. Osteoarticular allografts for reconstruction after resection of a musculoskeletal tumor in the proximal end of the tibia. J Bone Joint Surg Am. 1994;76:549–554.
Cristofolini L, Bini SA, Toni A. In vitro testing of a novel limb salvage prosthesis for the distal femur. Clin Biomech. 1998;13:608–615.
Damron TA. Endoprosthetic replacement following limb-sparing resection for bone sarcoma. Semin Surg Oncol. 1997;13:3–10.
Donati D, Colangeli M, Colangeli S, Di Bella C, Mercuri M. Allograft-prosthetic composite in the proximal tibia after bone tumor resection. Clin Orthop Relat Res. 2008;466:459–465.
Eckardt JJ, Matthews JG 2nd, Eilber FR. Endoprosthetic reconstruction after bone tumor resections of the proximal tibia. Orthop Clin North Am. 1991;22:149–160.
Flint MN, Griffin AM, Bell RS, Ferguson PC, Wunder JS. Aseptic loosening is uncommon with uncemented proximal tibial tumor prostheses. Clin Orthop Relat Res. 2006;450:52–59.
Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res. 2006;450:164–171.
Griffin AM, Parsons JA, Davis AM, Bell RS, Wunder JS. Uncemented tumor endoprostheses at the knee: root causes of failure. Clin Orthop Relat Res. 2005;438:71–79.
Grimer RJ, Carter SR, Tillman RM, Sneath RS, Walker PS, Unwin PS, Shewell PC. Endoprosthetic replacement of the proximal tibia. J Bone Joint Surg Br. 1999;81:488–494.
Henshaw RM, Bickels J, Malawer MM. Modular endoprosthetic reconstruction for lower extremity skeletal defects: oncologic and reconstructive indications. Semin Arthroplasty. 1999;10:180–187.
Horowitz SM, Lane JM, Otis JC, Healey JH. Prosthetic arthroplasty of the knee after resection of a sarcoma in the proximal end of the tibia: a report of sixteen cases. J Bone Joint Surg Am. 1991;73:286–293.
Jeys LM, Kulkarni A, Grimer RJ, Carter SR, Tillman RM, Abudu A. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Joint Surg Am. 2008;90:1265–1271.
Kawai A, Healey JH, Boland PJ, Athanasian EA, Jeon D-G. A rotating hinge knee replacement for malignant tumors of the femur and tibia. J Arthroplasty. 1999;14:187–196.
Kramer MJ, Tanner BJ, Horvai AE, O’Donnell RJ. Compressive osseointegration promotes viable bone at the endoprosthetic interface: retrieval study of Compress implants. Int Orthop. 2008;32:567–571.
Malawer MM. Limb-sparing surgery for malignant tumors of the proximal tibia. In: Sugarbaker PH, Malawer MM, eds. Musculoskeletal Surgery for Cancer. New York, NY: Thieme Medical Publishers, Inc; 1992:270–281.
Malawer MM, Chou LB. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcomas. J Bone Joint Surg Am. 1995;77:1154–1165.
Malawer MM, McHale KA. Limb-sparing surgery for high-grade malignant tumors of the proximal tibia: surgical technique and a method of extensor mechanism reconstruction. Clin Orthop Relat Res. 1989;239:231–248.
Mittermayer F, Krepler P, Dominkus M, Schwameis E, Sluga M, Heinzl H, Kotz R. Long-term followup of uncemented tumor endoprostheses for the lower extremity. Clin Orthop Relat Res. 2001;388:167–177.
Morgan HD, Cizik AM, Leopold SS, Hawkins DS, Conrad EU III. Survival of tumor megaprostheses replacements about the knee. Clin Orthop Relat Res. 2006;450:39–45.
Myers GJC, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br. 2007;89:521–526.
Myers GJC, Abudu AT, Carter SR, Tillman RM, Grimer RJ. The long-term results of endoprosthetic replacement of the proximal tibia for bone tumours. J Bone Joint Surg Br. 2007;89:1632–1637.
Natarajan MV, Sivaseelam A, Rajkumar Z, Hussain SHJ. Custom megaprosthetic replacement for proximal tibial tumours. Int Orthop. 2003;27:334–337.
O’Donnell RJ. Compressive osseointegration of modular endoprostheses. Curr Opin Orthop. 2007;18:590–603.
Ogihara Y, Sudo A, Fujinami S, Sato K. Limb salvage for bone sarcoma of the proximal tibia. Int Orthop. 1991;15:377–379.
Orlic D, Smerdelj M, Kolundzic R, Bergovec M. Lower limb salvage surgery: modular endoprosthesis in bone tumour treatment. Int Orthop. 2006;30:458–464.
Plötz W, Rechl H, Burgkart R, Messmer C, Schelter R, Hipp E, Gradinger R. Limb salvage with tumor endoprostheses for malignant tumors of the knee. Clin Orthop Relat Res. 2002;405:207–215.
Sanjay BKS, Moreau PG. Limb salvage surgery in bone tumour with modular endoprosthesis. Int Orthop. 1999;23:41–46.
Sharma S, Turcotte RE, Isler MH, Wong C. Cemented rotating hinge endoprosthesis for limb salvage of distal femur tumors. Clin Orthop Relat Res. 2006;450:28–32.
Sim FH, Beauchamp CP, Chao EYS. Reconstruction of musculoskeletal defects about the knee for tumor. Clin Orthop Relat Res. 1987;221:188–201.
Terek RM, Hulstyn MJ. Osteoarticular allograft reconstruction for tumors of the distal femur and proximal tibia. Oper Tech Orthop. 2005;14:236–242.
Torbert JT, Fox EJ, Hosalkar HS, Ogilvie CM, Lackman RD. Endoprosthetic reconstructions: results of long-term followup of 139 patients. Clin Orthop Relat Res. 2005;438:51–59.
Ward WG, Eckardt JJ, Johnston-Jones KS, Eilber FR, Namba R, Dorey FJ, Mirra J, Kabo JM. Five to ten year results of custom endoprosthetic replacement for tumors of the distal femur. In: Brown KLB, ed. Complications of Limb Salvage: Prevention, Management and Outcomes. Montreal, Quebec, Canada: International Society of Limb Salvage; 1991:483–491.
Ward WG, Johnston KS, Dorey FJ, Eckardt JJ. Extramedullary porous coating to prevent diaphyseal osteolysis and radiolucent lines around proximal tibial implants. J Bone Joint Surg Am. 1993;75:976–987.
Wirganowicz PZ, Eckardt JJ, Dorey FJ, Eilber FR, Kabo JM. Etiology and results of tumor endoprosthesis revision surgery in 64 patients. Clin Orthop Relat Res. 1999;358:64–74.
Wold LE, Adler C-P, Sim FH, Unni KK. Atlas of Orthopedic Pathology. Philadelphia, PA: Saunders; 2003.
Wunder JS, Leitch K, Griffin AM, Davis AM, Bell RS. Comparison of two methods of reconstruction for primary malignant tumors at the knee: a sequential cohort study. J Surg Oncol. 2001;77:89–99.
Yaw KM, Wurtz LD. Resection and reconstruction for bone tumors in the proximal tibia. Orthop Clin North Am. 1991;22:133–148.
Zeegan EN, Aponte-Tinao LA, Hornicek FJ, Gebhardt MC, Mankin HJ. Survivorship analysis of 141 modular metallic endoprostheses at early follow-up. Clin Orthop Relat Res. 2004;420:239–250.
Zhang Y, Yang Z, Li X, Chen Y, Zhang S, Du M, Li J. Custom prosthetic reconstruction for proximal tibial osteosarcoma with proximal tibiofibular joint involved. Surg Oncol. 2008;17:87–95.
Zwart HJ, Taminiau AH, Schimmel JW, van Horn JR. Kotz modular femur and tibia replacement: 28 tumor cases followed for 3 (1–8) years. Acta Orthop Scand. 1994;65:315–318.
Acknowledgments
I thank Philip J. Kruger, MD, for assistance with preparation of the abstract as well as Mark C. Gebhardt, MD, David G. Mohler, MD, and Scott D. Weiner, MD, for assistance with patient care and followup.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
The author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
The author certifies that his institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
O’Donnell, R.J. Compressive Osseointegration of Tibial Implants in Primary Cancer Reconstruction. Clin Orthop Relat Res 467, 2807–2812 (2009). https://doi.org/10.1007/s11999-009-0986-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-009-0986-4