Abstract
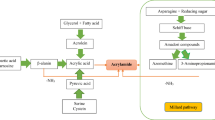
Acrylamide is a potential cause of a wide spectrum of toxic effects and is classified as probably “carcinogenic in humans”. The discovery of acrylamide in human foods has given rise to extensive studies exploring its formation mechanisms and levels of exposure and has spurred search into suitable analytical procedures for its determination in foodstuffs. However, the exact chemical mechanisms governing acrylamide formation are not yet known and cheap, convenient, and rapid screening methods are still to be developed. Acrylamide in food is produced by heat-induced reactions between the amino group of asparagine and the carbonyl group of reducing sugars along with thermal treatment of early Maillard reaction products (N-glycosides). Similarly, the decarboxylated Schiff base and decarboxylated Amadori compounds of asparagine as well as the Strecker aldehyde have been proposed as direct precursors and intermediates of acrylamide. Corresponding chromatographic methods are used to determine various structural groups present in Maillard reaction model systems. Gas chromatography-mass spectrometry and liquid chromatography with tandem mass spectrometry analysis are both acknowledged as the main, useful, and authoritative methods for acrylamide determination. This review is an attempt to summarize the state-of-the-art knowledge of acrylamide chemistry, formation mechanisms, and analytical methods. Special attention is given to comparison of different chromatographic techniques, particularly the novel, simple, and low-cost methods of its determination.




Similar content being viewed by others
References
Açar, Ö. Ç., Pollio, M., Monaco, R. D., Fogliano, V., & Gökmen, V. (2010). Effect of calcium on acrylamide level and sensory properties of cookies. Food and Bioprocess Technology. doi:10.1007/s11947-009-0317-5.
Ahn, J. S., Castle, L., Clarke, D. B., Lioyd, A. S., Philo, M. R., & Speck, D. R. (2002). Verification of findings of acrylamide in heated foods. Food Additives and Contaminants, 19, 1116–1124.
Andrawes, F., Greenhouse, S., & Draney, D. (1987). Chemistry of acrylamide bromination for trace analysis by gas chromatography and gas chromatography-mass spectrometry. Journal of Chromatography, 399, 269–275.
Andrzejewski, D., Roach, J. A. G., Gay, M. L., & Musser, S. M. (2004). Analysis of coffee for the presence of acrylamide by LC-MS/MS. Journal of Agricultural and Food Chemistry, 52, 1996–2002.
Arikawa, A., & Shiga, M. (1980). Determination of trace acrylamide in the crops by gas chromatography. Bunseki Kagaku, 29, T33–T39.
Basilicata, P., Miraglia, N., Pieri, M., Acampora, A., Soleo, L., & Sanolo, N. (2005). Application of the standard addition approach for the quantification of urinary benzene. Journal of Chromatography B, 818, 293–299.
Becalski A, Lau BPY, Lewis D & Seaman S (2002) Major pathway of formation of acrylamide in foods and possible approaches to mitigation. Abstracts of 116th AOAC Annual Meeting, Los Angeles, USA.
Becalski, A., Lau, B. P. Y., Lewis, D., & Seaman, S. W. (2003). Acrylamide in foods: Occurrence, sources, and modelling. Journal of Agricultural and Food Chemistry, 51, 802–808.
Biedermann, M., Biedermann-Brem, S., Noti, A., & Grob, K. (2002a). Methods for determining the potential of acrylamide formation and its elimination in raw materials for food preparation, such as potatoes. Mitteilungen aus Lebensmitteluntersuchung und Hygiene, 93, 653–667.
Biedermann, M., Biedermann-Brem, S., Noti, A., Grob, K., Egli, P., & Mandli, H. (2002b). Two GC-MS methods for the analysis of acrylamide in foods. Mitteilungen aus Lebensmitteluntersuchung und Hygiene, 93, 638–652.
Biedermann, M., Noti, A., Biedermann-Brem, S., Mozzetti, V., & Grob, K. (2002c). Experiments on acrylamide formation and possibilities to decrease the potential of acrylamide formation in potatoes. Mitteilungen aus Lebensmitteluntersuchung und Hygiene, 93, 668–687.
Bologna, L. S., Andrawes, F. F., Barvenik, F. W., Lentz, R. D., & Sojka, R. E. (1999). Analysis of residual acrylamide in field crops. Journal of Chromatography Science, 37, 240–244.
Borodin, N. (1925). Biological observations on the Atlantic sturgeon. Transactions of the American Fisheries Society, 55, 184–190.
Brandl, F., Demiani, S., Ewender, J., Franz, R., Gmeiner, M., Gruber, L., et al. (2002). A rapid and convenient procedure for the determination of acrylamide in foodstuffs. Electronic Journal of Environmental, Agricultural and Food Chemistry, 1(3), 137–144.
Brown, L., & Rhead, M. M. (1979). Liquid chromatographic determination of acrylamide monomer in natural and polluted aqueous environments. The Analyst, 104, 391–399.
Brown, L., Rhead, M. M., Hill, D., & Bancroft, K. C. C. (1982). Quantitative and quantitative studies on the in situ adsorption, degradation and toxicity of acrylamide by the spiking of the waters of two sewage works and a river. Water Research, 16, 579–591.
Casella, I. G., & Contursi, M. (2004). Quantitative analysis of acrolein in heated vegetable oils by liquid chromatography with pulsed electrochemical detection. Journal of Agricultural and Food Chemistry, 52, 5816–5821.
Castle, L. (1993). Determination of acrylamide monomer in mushrooms grown on polyacrylamide gel. Journal of Agricultural and Food Chemistry, 41, 1261–1263.
Castle L (2003) Determination of acrylamide in food: GC-MS, bromination method, Presentation at the Workshop Analytical Methods for Acrylamide Determination in Food', Belgium, Oud-Turnhout.
Castle, L., & Eriksson, S. (2005). Analytical methods used to measure acrylamide concentrations in foods. Journal of AOAC International, 88, 274–284.
Castle, L., Campos, M. J., & Gilbert, J. (1991). Determination of acrylamide monomer in hydroponically grown tomato fruits by capillary gas chromatography-mass spectrometry. Journal of the Science of Food and Agriculture, 54, 549–555.
Cavalli S, Maurer R & Höfler F (2002) Fast determination of acrylamide in food samples using accelerated solvent extraction followed by ion chromatography with UV or MS-detection, 2003, LC/GC Europe, The Applications book, pp. 1-3.
Cavalli, S., Polesello, S., & Saccani, G. (2004). Determination of acrylamide in drinking water by large-volume direct injection and ion-exclusion chromatography-mass spectrometry. Journal of Chromatography A, 1039, 155–159.
Claus, A., Carle, R., & Schieber, A. (2008). Acrylamide in cereal products: A review. Journal of Cereal Science, 47, 118–133.
Delatour, T., Périsset, A., Goldmann, T., Riedeker, S., & Stadler, R. H. (2004). Improved sample preparation to determine acrylamide in difficult matrixes such as chocolate powder, cocoa, and coffee by liquid chromatography tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 52, 4625–4631.
Dionex (2004) Fast determination of acrylamide in food samples using Accelerated Solvent Extraction (ASE) followed by Ion chromatography with UV or MS detection. Application Note, 409, 1-4.
Dunovska, L., Cajka, T., Hajslova, J., & Holadova, K. (2006). Direct determination of acrylamide in food by gas chromatography–high-resolution time-of-flight mass spectrometry. Analytica Chimica Acta, 578, 234–240.
Eberhart, B. L., Ewald, D. K., Sanders, R. A., Tallmadge, D. H., Zyzak, D. V., & Strothers, M. A. (2005). Quantitation of acrylamide in food products by liquid chromatography/mass spectrometry. Journal of AOAC International, 88, 1205–1211.
EEC, Commission Directive 92/39/EEC, amending Directive 90/128/EEC (1992) Relating to plastic materials and articles intended to come into contact with foodstuffs. Official Journal of the European Communities, L330, 21–29.
EEC, Council Directive 98/83/EC (1998) On the quality of water intended for human consumption. Official Journal of the European Communities, L330, 32–54.
Eriksson, S. (2005). Acrylamide in food products: identification, formation and analytical methodology. PhD Thesis. Stockholm University, Stockholm, Sweden.
Eriksson, S., & Karlsson, P. (2005). Some analytical factors affecting measured levels of acrylamide in food products. In M. Friedman & D. Mottram (Eds.), Chemistry and safety of acrylamide in food (pp. 285–291). New York: Springer Science and Business Media, Inc.
European Union Risk Assessment Report (2002) Acrylamide, EUR 19835 EN, 24, pp. 1-207, Office for Official Publications of the European Communities, Luxembourg.
FAO/WHO (2004) Discussion paper on acrylamide. Thirty-sixth Session Rotterdam, The Netherlands, 22–26 March.
Fauhl, C. (2003). Proficiency testing acrylamide, Presentation at the Workshop Analytical Methods for Acrylamide Determination in Food. Oud-Turnhout: Belgium.
Fauhl C, Klaffke H, Mathar W, Palvinskas R & Wittkowski R (2002) Acrylamide interlaboratory study 2002, (http://www.bfr.bund.de/cms/detail.php?template=internet_de_index_is)
Friedman, M. (1978). Inhibition of lysinoalanine synthesis by protein acylation. Advances in Experimental Medicine and Biology, 105, 613–648.
Friedman, M. (1996). Food browning and its prevention: an overview. Journal of Agriculture and Food Chemistry, 44, 631–653.
Friedman, M. (1997). Chemistry, biochemistry, and dietary role of potato polyphenols. A review. Journal of Agriculture and Food Chemistry, 45, 1523–1540.
Friedman, M. (2001). Nutritional and health benefits of soy proteins. Journal of Agriculture and Food Chemistry, 49, 1069–1086.
Genga, Z., Jiang, R., & Chena, M. (2008). Determination of acrylamide in starch-based foods by ion-exclusion liquid chromatography. Journal of Food Composition and Analysis, 21, 178–182.
Gertz, C., & Klostermann, S. (2002). Analysis of acrylamide and mechanisms of its formation in deep-fried products. European Journal of Lipid Science and Technology, 104, 762–771.
Gökmen, V., & Palazoğlu, T. K. (2008). Acrylamide formation in foods during thermal processing with a focus on frying. Food and Bioprocess Technology, 1, 35–42.
Gökmen, V., Senyuva, H. Z., Acar, J., & Sarioglu, K. (2005). Determination of acrylamide in potato chips and crisps by high-performance liquid chromatography. Journal of Chromatography A, 1088, 193–199.
Gökmen, V., Morales, F. J., Atac, B., Serpen, A., & Arribas-Lorenzo, G. (2009). Multiple-stage extraction strategy for the determination of acrylamide in foods. Journal of Food Composition and Analysis, 22, 142–147.
Granvogl, M., Jezussek, M., Koehler, P., & Schieberle, P. (2004). Quantification of 3-aminopropionamide in potatoes, a minor but potent precursor in acrylamide formation. Journal of Agricultural and Food Chemistry, 52, 4751–4757.
Grigg, R., & Thianpatanagul, S. (1984). Decarboxylative transamination, mechanism and application to the synthesis of heterocyclic compounds. Journal of the Chemical Society, Chemical Communications, 180-181.
Grigg, R., Surendrakumar, S., Thianpatanagul, S., & Vipond, D. (1988). X = Y-ZH systems as potential 1, 3-dipoles, Part 11, stereochemistry of 1, 3-dipoles generated by the decarboxylative route to azomethine ylides. Journal of the Chemical Society Perkin Transactions, 1, 2693–2701.
Hamdan, M., Bordini, E., Galvani, M., & Righetti, P. G. (2001). Isotope-coded two-dimensional maps: Tagging with deuterated acrylamide and 2-vinylpyridine. Electrophoresis, 22, 1633–1644.
Hamlet, C. G., Jayaratne, S. M., & Sadd, P. A. (2004). Rapid, sensitive and selective analysis of acrylamide in cereal products using bromination and GC/MS/MS. Czech Journal of Food Sciences, 22, 290–293.
Hashimoto, A. (1976). Improved method for the determination of acrylamide monomer in water by means of gas-liquid chromatography with an electron capture detector. The Analyst, 101, 932–938.
Hoenicke, K., & Gatermann, R. (2004). Stability of acrylamide in food during storage. Czech Journal of Food Sciences, 22, 355–356.
Hoenicke, K., & Gatermann, R. (2005). Studies on the stability of acrylamide in food during storage. Journal of AOAC International, 88, 268–273.
Hoenicke, K., Gatermann, R., Harder, W., & Hartig, L. (2004). Analysis of acrylamide in different foodstuffs using liquid chromatography–tandem mass spectrometry and gas chromatography–tandem mass spectrometry. Analytica Chimica Acta, 520, 207–215.
Höfler, F., Maurer, R., & Cavalli, S. (2002). Schnelle Analyse von Acrylamid in Lebensmitteln mit ASE und LC/MS. GIT Labor-Fachzeitschrift, 9, 968–970.
IARC (1994) Acrylamide, In IARC monographs on the evaluation of carcinogenic risks to humans, some industrial chemicals, 60, pp. 389-433, WHO, Geneva, Switzerland.
Ito, S., & Tsukada, K. (2001). Matrix effect and correction by standard addition in quantitative liquid chromatographic-mass spectrometric analysis of diarrhetic shellfish poisoning toxins. Journal of Chromatography A, 943, 39–46.
Kawata, K., Ibaraki, T., Tanabe, A., Yagoh, H., Shinoda, A., Suzuki, H., et al. (2001). Gas chromatographic-mass spectrometric determination of hydrophilic compounds in environmental water by solid-phase extraction with activated carbon fiber felt. Journal of Chromatography A, 911, 75–83.
Keyhani, A., & Yaylayan, V. A. (1996). Pyrolysis/GC/MS analysis of N-(1-deoxy-d-fructose-1-yl)-l-phenylalanine: Identification of novel pyridine and naphthalene derivatives. Journal of Agricultural and Food Chemistry, 44, 223–229.
Kim, Y., Faqih, M. N., & Wang, S. S. (2001). Factors affecting gel formation of inulin. Carbohydrate Polymers, 46, 135–145.
Kim, C. T., Hwang, E. S., & Lee, H. J. (2007). An improved LC-MS/MS method for the quantitation of acrylamide in processed foods. Food Chemistry, 101, 401–409.
Kroh, L. W. (1994). Caramelisation in food and beverages. Food Chemistry, 51, 373–379.
Ledl, F., & Schleicher, E. (1990). New aspects of Maillard reaction in foods and in the human body. Angewandte Chemie International Edition in English, 29, 565–594.
Lee, M. R., Chang, L. Y., & Dou, J. (2007). Determination of acrylamide in food by solid-phase microextraction coupled to gas chromatography–positive chemical ionization tandem mass spectrometry. Analytica Chimica Acta, 582, 19–23.
Liu, J., Zhao, G., Yuan, Y., Chen, F., & Hu, X. (2008). Quantitative analysis of acrylamide in tea by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Food Chemistry, 108, 760–767.
Magnin, E. (1964). Validité d’une distinction spécifique entre les deux acipenseridés: Acipenser sturio L. d’ Europe et Acipenser oxyrhynchus d’Amerique du Nord. Naturaliste Canadien, 91, 5–20.
Manini, P., d’Ischina, M., & Prota, G. (2001). An unusual decarboxylative Maillard reaction between L-DOPA and D-glucose under biomimetic conditions: Factors governing with Pictet-Spengler condensation. The Journal of Organic Chemistry, 66, 5048–5053.
Manson, J., Brabec, M. J., Buelke-Sam, J., Carlson, G. P., Chapin, R. E., Favor, J. B., et al. (2005). NTP-CERHR expert panel report on the reproductive and developmental toxicity of acrylamide. Birth Defects Research (Part B), 74, 17–113.
Martin, E., Samec, J., & Vogel, J. (1990). Détermination de l’acrylamide dans l’eau par chromatographie en phase gazeuse (GC). Travaux de Chimie Alimentaire et d’Hygiéne, 81, 327–330 (In French).
Mestdagh, F., De Meulenaer, B., Van Peteghem, C., Cromphout, C., & Thas, O. (2004). Towards a better understanding in acrylamide formation, degradation and reduction in model systems (and foodstuffs). Czech Journal of Food Sciences, 22, 11–14.
Mottram, D. S., Wedzicha, B. L., & Dodson, A. T. (2002). Acrylamide is formed in the Maillard reaction. Nature, 419, 448.
Nemoto, S., Takatsuki, S., Sasaki, K., & Maitani, T. (2002). Determination of acrylamide in foods by GC/MS using 13C-labeled acrylamide as an internal standard. Journal of the Food Hygienic Society of Japan, 43, 371–376.
Ono, H., Chuda, Y., Ohnishi-Kameyama, M., Yada, H., Ishizaka, M., Kobayashi, H., et al. (2003). Analysis of acrylamide by LC-MS/MS and GC-MS in processed Japanese foods. Food Additives and Contaminants, 20, 215–220.
Pedreschi, F., Granby, K., & Risum, J. (2010). Acrylamide mitigation in potato chips by using NaCl. Food and Bioprocess Technology. doi:10.1007/s11947-010-0349-x.
Pérez, H. L., & Osterman-Golkar, S. (2003). A sensitive gas chromatographic-tandem mass spectrometric method for detection of alkylating agents in water: Application to acrylamide in drinking water, coffee and snuff. The Analyst, 128, 1033–1036.
Pittet, A., Périsset, A., & Oberson, J. M. (2004). Trace level determination of acrylamide in creal-based foods by gas chromatography-mass spectrometry. Journal of Chromatography A, 1035, 123–130.
Poole, C. F. (1981). Determination of acrylamide in nerve tissue homogenates by electron capture gas chromatography. Journal of Chromatography A, 217, 239–245.
Press release: HEATOX project completed-brings new pieces to the acrylamide puzzle (2007) Livsmedelsverket, pp. 9-19.
Ramírez-Jiménez, A., Guerra-Hernández, E., & García-Villanova, B. (2000). Browning indicators in bread. Journal of Agricultural and Food Chemistry, 48, 4176–4181.
Ren, Y., Zhang, Y., Jiao, J., Cali, Z., & Zhang, Y. (2006). Sensitive isotope dilution liquid chromatography/electrospray ionization tandem mass spectrometry method for the determination of acrylamide in chocolate. Food Additives and Contaminants, 23, 228–236.
Results of a BgVV information seminar (2002) Acrylamide in foods—serious problem or exaggerated risk?, Bundesinstitut, für gesundheitlichen Verbraucherschutz und Veterinärmedizin.
Richter, B. E., Jones, B. A., Ezzell, J. L., Porter, N. L., Avdalovic, N., & Pohl, C. (1996). Accelerated solvent extraction: A technique for sample preparation. Analytical Chemistry, 68, 1033–1039.
Riediker, S., & Stadler, R. H. (2003). Analysis of acrylamide in food by isotope-dilution liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Journal of Chromatography A, 1020, 121–130.
Roach, J. A. G., Andrzejewski, D., Gay, M. L., Nortrup, D., & Musser, S. M. (2003). Rugged LC-MS/MS survey analysis for acrylamide in foods. Journal of Agriculture and Food Chemistry, 51, 7547–7554.
Robert, F., Vuataz, G., Pollien, P., Saucy, F., Alonso, M. I., Bauwens, I., et al. (2004). Acrylamide formation from asparagine under low-moisture Maillard reaction conditions, 1. Physical and chemical aspects in crystalline model systems. Journal of Agricultural and Food Chemistry, 52, 6837.
Rosén, J., & Hellenäs, K. E. (2002). Analysis of acrylamide in cooked foods by liquid chromatography tandem mass spectrometry. The Analyst, 127, 880–882.
Rothweiler, B., & Prest, H. (2003). Rapid screening for acrylamide in foods using GC-MS with positive chemical ionization, LC-GC, application notebook 34.
Sanders, R. A., Zyzak, D. V., Stojanovic, M., Tallmadge, D. H., Eberhart, B. L., & Ewald, D. K. (2002). An LC-MS acrylamide method and its use in investigating the role of asparagines. Acrylamide Symposium, Abstracts of 116th Annual AOAC International Meeting, Los Angeles, USA.
Schabacker, J., Schwend, T., & Wink, M. (2004). Reduction of acrylamide uptake by dietary proteins in a CaCo-2 gut model. Journal of Agriculture and Food Chemistry, 52, 4021–4025.
Schaller, U. (2003). Experiences with acrylamide determination view from a retailer’s laboratory, Presentation at the workshop “Analytical methods for acrylamide determination in food”, Oud-Turnhout, Belgium.
Schönberg, A., & Moubacher, R. (1952). The Strecker degradation of α-amino acids. Chemical Reviews, 50, 261–277.
Scientific Committee on Food (SCF) (2002). Opinion of the Scientific Committee on Food on new findings regarding the presence of acrylamide in food, SCF/CS/CNTM/CONT/4 Final.
Serpen, A., & Gokmen, V. (2007). Modelling of acrylamide formation and browning ratio in potato chips by artificial neural network. Molecular Nutrition & Food Research, 51, 383–389.
Sohn, M., & Ho, C. T. (1995). Ammonia generation during thermal degradation of amino acids. Journal of Agricultural and Food Chemistry, 43, 3001–3003.
Stadler, R. H., Blank, I., Varga, N., Robert, F., Hau, J., Guy, P. A., et al. (2002). Acrylamide from Maillard reaction products. Nature, 419, 449.
Stadler, R. H., Verzegnassi, L., Varga, N., Grigorov, M., Studer, A., Riediker, S., et al. (2003). Formation of vinylogous compounds in model Maillard reaction systems. Chemical Research in Toxicology, 16, 1242–1250.
Stadler, R. H., Robert, F., Riediker, S., Varga, N., Davidek, T., Devaud, S., et al. (2004). In-depth mechanistic study on the formation of acrylamide and other vinylogous compounds by the Maillard reaction. Journal of Agricultural and Food Chemistry, 52, 5550–5558.
Svensson, K., Abramsson, L., Becker, W., Glynn, A., Hellenäs, K. E., Lind, Y., et al. (2003). Dietary intake of acrylamide in Sweden. Food and Chemical Toxicology, 41, 1581–1586.
Swiss Federal Office of Public Health (2002) Determination of acrylamide in food, [http://www.bag.admin.ch/verbrau/aktuell/d/AA_methode].
Taeyman, D., Wood, J., Ashby, B., Blank, I., Studer, A., Stadler, R. H., et al. (2004). A review of acrylamide: an industry perspective on research, analysis, formation, and control. Critical Reviews in Food Science and Nutrition, 44, 323–347.
Takata, K., & Okamoto, T. (1991). A method for determination of acrylamide in environmental samples by gas chromatography using bromination-dehydrobromination. Kankyo Kagaku, 1, 559–565.
Takatsuki, S., Nemoto, S., Sasaki, K., & Maitani, T. (2003). Determination of acrylamide in processed foods by LC/MS using column switching. Journal of the Food Hygienic Society of Japan, 44, 89–95.
Tareke, E., Rydberg, P., Karlsson, P., Eriksson, S., & Törnqvist, M. (2000). Acrylamide: a cooking carcinogen? Chemical Research in Toxicology, 13, 517–522.
Tareke, E., Rydberg, P., Karlsson, P., Eriksson, S., & Törnqvist, M. (2002). Analysis of acrylamide, a carcinogen formed in heated foodstuffs. Journal of Agricultural and Food Chemistry, 50, 4998–5006.
Tateo, E., & Bononi, M. (2003). A GC/MS method for the routine determination of acrylamide in food. Italian Journal of Food Science, 15, 149–151.
Taubert, D., Harlfinger, S., Henkes, L., Berkels, R., & Schömig, E. (2004). Influence of processing parameters on acrylamide formation during frying of potatoes. Journal of Agricultural and Food Chemistry, 52, 2735–2739.
Tekel, J., Farkaš, P., & Kováč, M. (1989). Determination of acrylamide in sugar by capillary GLC with alkali flame-ionization detection. Food Additives and Contaminants, 6, 377–381.
Terada, H., & Tamura, Y. (2003). Determination of acrylamide in processed foods by column-switching HPLC with UV detection. Journal of the Food Hygienic Society of Japan, 44(6), 303–309.
Törnqvist, M. (2005). Acrylamide in food: the discovery and its implications. In M. Friedman & D. Mottram (Eds.), Chemistry and safety of acrylamide in food (pp. 1–19). New York: Springer Science and Business Media, Inc.
United States Environmental Protection Agency (U.S. EPA) (1996). Method 8032A, Acrylamide by gas chromatography. In: SW 846, Test methods for evaluating solid waste, pp. 1–14. Washington, DC, USA.
US Food and Drug Administration (FDA) (2003) Draft: Detection and quantitation of acrylamide in foods [http://vm.cfsan.fda.gov/~dms/acrylami.html].
Vattem, D. A., & Shetty, K. (2003). Acrylamide in food: a model for mechanism of formation and its reduction. Innovative Food Science & Emerging Technologies, 4, 331.
Weibhaar, R. (2004). Acrylamide in heated potato products, analystic and formation routs. European Journal of Lipid Science and Technology, 106, 786–792.
Weibhaar, R., & Gutsche, B. (2002). Formation of acrylamide in heated potato products, model experiments pointing to asparagine as precursor. Deutsche Lebensmittel-Rundschau, 98, 397–400.
Wenzl, T., Beatriz de la Calle, M., & Anklam, E. (2003). Analytical methods for the determination of acrylamide in food products: a review. Food Additives and Contaminants, 20(10), 885–902.
Wiertz-Eggert-Jörissen (WEJ) GmbH (2003) Standard operation procedure for the determination of acrylamide in baby food. Standard Operation Procedure (SOP) WEJ GmbH, Hamburg, Germany.
Wnorowski, A., & Yaylayan, V. A. (2000). Influence of pyrolytic and aqueous phase reactions on the mechanism of formation of Maillard products. Journal of Agricultural and Food Chemistry, 48, 3549–3554.
Wnorowski, A., & Yaylayan, V. A. (2003). Monitoring carbonyl-amine reaction between pyruvic acid and α-amino alcohols by FTIR spectroscopy, a possible route to Amadori products. Journal of Agricultural and Food Chemistry, 51, 6537–6543.
World Health Organisation (WHO) (1996) Guidelines for Drinking-Water Quality, 2nd edition, Vol. 2, pp. 940-949.
Yang, J., Powers, J. R., Boylston, T. D., & Weller, K. M. (1999). Sugars and free amino acids in stored russet Burbank potatoes treated with CIPC and alternative sprout inhibitors. Journal of Food Science, 64, 592–596.
Yasuhara, A., Tanaka, Y., Hengel, M., & Shibamoto, T. (2003). Gas chromatographic investigation of acrylamide formation in browning model systems. Journal of Agricultural and Food Chemistry, 51, 3999–4003.
Yaylayan, V. A. (1999). Analysis of complex reaction mixtures: Novel applications of Py-GC/MS and Microwave Assisted Synthesis (MAS). American Laboratory, 31(9), 30–31.
Yaylayan, V. A., Harty-Majors, S., & Ismail, A. (1999a). Investigation of dl-glyceraldehyde-dihydroxyacetone interconversion by FTIR spectroscopy. Carbohydrate Research, 318, 34–39.
Yaylayan, V. A., Harty-Majors, S., & Ismail, A. A. (1999b). Monitoring carbonyl amine reaction and enolization of 1-hydroxy-2-propanone by FTIR spectroscopy. Journal of Agricultural and Food Chemistry, 47, 2335–2340.
Yaylayan, V. A., Wnorowski, A., & Perez Locas, C. (2003). Why asparagine needs carbohydrates to generate acrylamide. Journal of Agricultural and Food Chemistry, 51, 1753–1757.
Yaylayan, V. A., Perez Locas, C., Wnorowski, A., & O’Brien, J. (2004). The role of creatine in the generation of N-methylacrylamide: A new toxicant in cooked meat. Journal of Agricultural and Food Chemistry, 52, 5559–5565.
Yaylayan, V. A., Perez Locas, C., Wnorowski, A., & O’Brien, J. (2005). Mechanistic pathways of formation of acrylamide from different amino acids. In Friedman & Mottram (Eds.), Chemistry and safety of acrylamide in food (pp. 191–203). New York: Springer and Business Media, Inc.
Zhang, Y., & Zhang, Y. (2007). Formation and reduction of acrylamide in Maillard reaction: A review based on the current state of knowledge. Critical Reviews in Food Science and Nutrition, 47, 521–542.
Zhang, Y., Zhang, G., & Zhang, Y. (2005). Occurrence and analytical methods of acrylamide in heat-treated foods: Review and recent developments. Journal of Chromatography A, 1075, 1–21.
Zhang, Y., Dong, Y., Ren, Y., & Zhang, Y. (2006). Rapid determination of acrylamide contaminant in conventional fried foods by gas chromatography with electron capture detector. Journal of Chromatography A, 1116, 209–216.
Zhang, Y., Ren, Y., Zhao, H., & Zhang, Y. (2007). Determination of acrylamide in Chinese traditional carbohydrate-rich foods using gas chromatography with micro-electron capture detector and isotope dilution liquid chromatography combined with electrospray ionization tandem mass spectrometry. Analytica Chimica Acta, 584, 322–332.
Zhu, Y., Li, G., Duan, Y., Chen, S., Zhang, C., & Li, Y. (2008). Application of the standard addition method for the determination of acrylamide in heat-processed starchy foods by gas chromatography with electron capture detector. Food Chemistry, 109, 899–908.
Zyzak, D. V., Sandres, R. A., Stojanovic, M., Tallmadge, D. H., Eberhart, B. L., Ewald, D. K., et al. (2003). Acrylamide formation mechanism in heated foods. Journal of Agricultural and Food Chemistry, 51, 4782–4787.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keramat, J., LeBail, A., Prost, C. et al. Acrylamide in Foods: Chemistry and Analysis. A Review. Food Bioprocess Technol 4, 340–363 (2011). https://doi.org/10.1007/s11947-010-0470-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-010-0470-x