Abstract
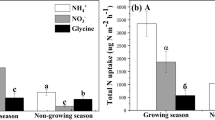
Due to their particular physiology and life history traits, bryophytes are critical in regulating biogeochemical cycles and functions in alpine ecosystem. Hence, it is crucial to investigate their nutrient utilization strategies in comparison with vascular plants and understand their responses to the variation of growing season caused by climate change. Firstly, this study testified whether or not bryophytes can absorb nitrogen (N) directly from soil through spiking three chemical forms of 15N stable isotope tracer. Secondly, with stronger ability of carbohydrates assimilation and photosynthesis, it is supposed that N utilization efficiency of vascular plants is significantly higher than that of bryophytes. However, the recovery of soil N by bryophytes can still compete with vascular plants due to their greater phytomass. Thirdly, resource acquisition may be varied from the change of growing season, during which N pulse can be manipulated with 15N tracer addition at different time. Both of bryophytes and vascular plants contain more N in a longer growing season, and prefer inorganic over organic N. Bryophytes assimilate more NH4+ than NO3- and amino acid, which can be indicated from the greater shoot excess 15N of bryophytes. However, vascular plants prefer to absorb NO3- for their developed root systems and vascular tissue. Concerning the uptake of three forms N by bryophytes, there is significant difference between two manipulated lengths of growing season. Furthermore, the capacity of bryophytes to tolerate N-pollution may be lower than currently appreciated, which indicates the effect of climate change on asynchronous variation of soil N pools with plant requirements.
Similar content being viewed by others
Reference
Aerts R, Cornelissen JHC, Dorrepaal E (2006) Plant performance in a warmer world: General responses of plants from cold, northern biomes and the importance of winter and spring events. Plant Ecology 182:65–77. DOI: 10.1007/s11258-005-9031-1.
Atkin OK, Botman B, Lambers H (1996) The relationship between the relative growth rate and nitrogen economy of alpine and lowland Poa species. Plant Cell and Environment 19:1324–1330.
Ayres E, van der Wal R, Sommerkorn M, et al. (2006) Direct uptake of soil nitrogen by mosses. Biology Letters 2:286–288. DOI: 10.1098/rsbl.2006.0455.
Badeck FW, Bondeau A, Bottcher DK, et al. (2004) Responses of spring phenology to climate change. New Phytologist 162:295–309. DOI: 10.1111/j.1469-8137.2004.01059.x.
Bardgett RD, van der Wal R, Jonsdottir IS, et al. (2007) Temporal variability in plant and soil nitrogen pools in a high-Arctic ecosystem. Soil Biology & Biochemistry 39:2129–2137. DOI: 10.1016/j.soilbio.2007.03.016
Beringer J, Lynch AH, Chapin FS, et al. (2001) The representation of arctic soils in the land surface model: The importance of mosses. Journal of Climate 14:3324–3335. DOI: 10.1175/1520-0442(2001)014〈3324:troasi〉2.0.co;2.
Blok D, Heijmans MM, Schaepman-Strub G, et al. (2011). The Cooling Capacity of Mosses: Controls on Water and Energy Fluxes in a Siberian Tundra Site. Ecosystems 14:1055–1065.
Cambui CA, Svennerstam H, Gruffman L, et al. (2011). Patterns of Plant Biomass Partitioning Depend on Nitrogen Source. Plos One 6. DOI: 10.1007/s10021-011-9463-5.
Cardon ZG, Gage DJ (2006) Resource exchange in the rhizosphere: Molecular tools and the microbial perspective. Pages 459–488 Annual Review of Ecology Evolution and Systematics. DOI: 10.1146/annurev.ecolsys.37.091305.110207.
Cassman KG, Munns DN (1980) Nitrogen mineralization as affected by soil-moisture, temperature, and depth. Soil Science Society of America Journal 44:1233–1237.
Chapin FS (1980) The Mineral-Nutrition of Wild Plants. Annual Review of Ecology and Systematics 11:233–260. DOI: 10.1146/annurev.es.11.110180.001313.
Chapin FS, McGuire AD, Randerson J, et al. (2000) Arctic and boreal ecosystems of western North America as components of the climate system. Global Change Biology 6:211–223. DOI: 10.1046/j.1365-2486.2000.06022.x.
Chmielewski FM, Rotzer T (2001) Response of tree phenology to climate change across Europe. Agricultural and Forest Meteorology 108:101–112.DOI:10.1016/s0168-1923(01)00233-7.
Cleland EE, Chiariello NR, Loarie SR, et al. (2006). Diverse responses of phenology to global changes in a grassland ecosystem. Proceedings of the National Academy of Sciences of the United States of America 103:13740–13744. DOI: 10.1073/pnas.0600815103.
Cole L, Buckland SM, Bardgett RD (2008) Influence of disturbance and nitrogen addition on plant and soil animal diversity in grassland. Soil Biology & Biochemistry 40:505–514. DOI: 10.1016/j.soilbio.2007.09.018.
DeLuca TH, Zackrisson O, Nilsson MC, et al. (2002) Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 419:917–920. DOI: 10.1038/nature01051.
Diaz S, Cabido M (1997) Plant functional types and ecosystem function in relation to global change. Journal of Vegetation Science 8:463–474. DOI: 10.2307/3237198.
Elser JJ, Bracken M, Cleland EE, et al. (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters 10:1135–1142. DOI: 10.1111/j.1461-0248.2007.01113.x.
Eskelinen A, Stark S, Mannisto M (2009) Links between plant community composition, soil organic matter quality and microbial communities in contrasting tundra habitats. Oecologia 161:113–123. DOI: 10.1007/s00442-009-1362-5.
Gornall JL, Jonsdottir IS, Woodin SJ, et al. (2007) Arctic mosses govern below-ground environment and ecosystem processes. Oecologia 153:931–941. DOI: 10.1007/s00442-007-0785-0.
Haynes RJ, Goh KM (1978) Ammonium and nitrate nutrition of plants. Biological Reviews of the Cambridge Philosophical Society 53:465–510.DOI: 10.1111/j.1469-185X.1978.tb00862.x.
Jaeger CH, Monson RK, Fisk MC, et al. (1999) Seasonal partitioning of nitrogen by plants and soil microorganisms in an alpine ecosystem. Ecology 80:1883–1891. DOI: 10.1890/0012-9658(1999)080[1883:sponbp]2.0.co;2.
Jauhiainen J, Wallen B, Malmer N (1998) Potential NH4+ and NO3- uptake in seven Sphagnum species. New Phytologist 138:287–293. DOI: 10.1046/j.1469-8137.1998.00110.x
Jones DL, Shannon D, Murphy DV, et al. (2004). Role of dissolved organic nitrogen (DON) in soil N cycling in grassland soils. Soil Biology & Biochemistry 36:749–756. DOI: 10.1016/j.soilbio.2004.01.003.
Körner C (1994) Impact of atmospheric changes on high mountain vegetation, in M. Beniston (ed.), Mountain Environments in Changing climates, Routledge, London. pp 155–166.
Körner C (2003) Alpine plant life: functional plant ecology of high mountain ecosystems. 2nd edition. Springer, Berlin; New York.
Kahmen A, Renker C, Unsicker SB (2006) Niche complementarity for nitrogen: An explanation for the biodiversity and ecosystem functioning relationship? Ecology 87:1244–1255.DOI:10.1890/0012-9658(2006)87[1244:ncfnae]2.0.co;2.
Kelly AE, Goulden ML (2008) Rapid shifts in plant distribution with recent climate change. Proceedings of the National Academy of Sciences of the United States of America 105:11823–11826. DOI: 10.1073/pnas.0802891105.
Kielland K (1994) Amino-acid-absorption by arctic plantsimplications for plant nutrition and nitrogen cycling. Ecology 75:2373–2383. DOI: 10.2307/1940891.
Kielland K (1995) Landscape patterns of free amino acids in arctic tundra soils. Biogeochemistry 31:85–98.
Krab EJ, Cornelissen JHC, Lang SI, et al. (2008) Amino acid uptake among wide-ranging moss species may contribute to their strong position in higher-latitude ecosystems. plant and soil 304:199–208. DOI: 10.1007/s11104-008-9540-5.
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379. DOI: 10.1890/06-2057.1.
Lindo Z, Gonzalez A (2010) The Bryosphere: an integral and influential component of the earth’s biosphere. Ecosystems 13:612–627. DOI: 10.1007/s10021-010-9336-3.
Malmer N, Svensson BM, Wallen B (1994). Interactions between sphagnum moses and field layer vascular plants in the development of peat-forming systems. folia geobotanica & phytotaxonomica 29:483–496.
Margeson JH, Suggs JC, Midgett MR (1980) Reduction of nitrate to nitrite with cadmium. Analytical Chemistry 52:1955–1957. DOI: 10.1021/ac50062a039.
McKane RB, Johnson LC, Shaver GR, et al. (2002) Resourcebased niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415:68–71. DOI: 10.1038/415068a.
Miller AJ, Cramer MD (2005) Root nitrogen acquisition and assimilation. Plant and Soil 274:1–36. DOI: 10.1007/s11104-004-0965-1.
Nilsson MC, Wardle DA, Zackrisson O, et al. (2002) Effects of alleviation of ecological stresses on an alpine tundra community over an eight-year period. Oikos 97:3–17. DOI: 10.1034/j.1600-0706.2002.970101.x.
Nord EA, Lynch JP (2009) Plant phenology: a critical controller of soil resource acquisition. Journal of Experimental Botany 60:1927–1937. DOI: 10.1093/jxb/erp018.
Oberbauer SF, Starr G, Pop EW (1998) Effects of extended growing season and soil warming on carbon dioxide and methane exchange of tussock tundra in Alaska. Journal of Geophysical Research: Atmospheres (1984–2012) 103:29075–29082.
Penuelas J, Filella I (2001) Phenology — Responses to a warming world. Science 294:793–795. DOI: 10.1126/science.1066860.
Persson J, Hogberg P, Ekblad A, et al. (2003) Nitrogen acquisition from inorganic and organic sources by boreal forest plants in the field. Oecologia 137:252–257.
Potter JA, Press MC, Callaghan TV, et al. (1995) Growth responses of Polytrichum commune and Hylocomium splendens to simulated environmental change in the subarctic. New Phytologist 131:533–541. DOI: 10.1007/s00442-003-1334-0.
Pouliot R, Marchand-Roy M, Rochefort L, et al. (2010) Estimating moss growth in arctic conditions: a comparison of three methods. Bryologist 113:322–332. DOI: 10.1639/0007-2745-113.2.322.
Raab TK, Lipson DA, Monson RK (1996) Non-mycorrhizal uptake of amino acids by roots of the alpine sedge Kobresia myosuroides: Implications for the alpine nitrogen cycle. Oecologia 108:488–494. DOI: 10.1007/bf00333725.
Raab TK, Lipson DA, Monson RK (1999). Soil amino acid utilization among species of the Cyperaceae: plant and soil processes. Ecology 80:2408–2419. DOI: 10.1890/0012-9658(1999)080[2408:saauas]2.0.co;2.
Rengel Z (1993) Mechanistic simulation models of nutrient uptake-a review. Plant and Soil 152:161–173. DOI: 10.1007/bf00029086.
Rich PM, Breshears DD, White AB (2008) Phenology of mixed woody-herbaceous ecosystems following extreme events: Net and differential responses. Ecology 89:342–352.DOI: 10.1890/06-2137.1.
Rustad LE, Campbell JL, Marion GM, et al (2001) A metaanalysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562.
Schimel JP, Bennett J (2004) Nitrogen mineralization: Challenges of a changing paradigm. Ecology 85:591–602. DOI: 10.1890/03-8002.
Shaver GR, Kummerow J (1992). Phenology, resource allocation, and growth of arctic vascular plants. Arctic ecosystems in a changing climate: an ecophysiological perspective: 193–211.
Sherry RA, Zhou X, Gu S, et al. (2007) Divergence of reproductive phenology under climate warming. Proceedings of the National Academy of Sciences of the United States of America 104:198–202. DOI: 10.1073/pnas.0605642104.
Smith MD, Knapp AK, Collins SL (2009) A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 90:3279–3289. DOI: 10.1890/08-1815.1.
Solheim B, Endal A, Vigstad H (1996) Nitrogen fixation in Arctic vegetation and soils from Svalbard, Norway. Polar Biology 16:35–40.
Steltzer H, Post E (2009) Seasons and Life Cycles. Science 324:886–887. DOI: 10.1007/bf02388733.
Thuiller W (2007) Biodiversity-Climate change and the ecologist. Nature 448:550–552. DOI: 10.1038/448550a.
Turetsky MR (2003) The role of bryophytes in carbon and nitrogen cycling. Bryologist 106:395–409. DOI: 10.1639/05.
Tylianakis JM, Didham RK, Bascompte J, et al. (2008). Global change and species interactions in terrestrial ecosystems. Ecology Letters 11:1351–1363. DOI: 10.1111/j.1461-0248.2008.01250.x.
Vantooren BF, Vandam D, During HJ (1990) The Relative Importance of Precipitation and Soil as Sources of Nutrients for Calliergonella-Cuspidata (Hedw) Loeske in Chalk Grassland. Functional Ecology 4:101–107.
Vitousek PM, Aber JD, Howarth RW, et al. (1997) Human alteration of the global nitrogen cycle: Sources and consequences. Ecological Applications 7:737–750. DOI: 10.2307/2269431.
Vitousek PM and Howarth RW (1991) Nitrogen Limitation on Land and in the Sea — How Can It Occur? Biogeochemistry 13:87–115.
vonWiren N, Gazzarrini S, Frommer WB (1997) Regulation of mineral nitrogen uptake in plants. Plant and Soil 196:191–199. DOI: 10.1023/a:1004241722172.
Woodward FI, Cramer W (1996) Plant functional types and climatic changes: Introduction. Journal of Vegetation Science 7:306–308.
Xu XL, Ouyang H, Richter W, et al. (2011) Spatio-temporal variations determine plant-microbe competition for inorganic nitrogen in an alpine meadow. Journal of Ecology 99:563–571. DOI: 10.1111/j.1365-2745.2010.01789.x.
Zaman M, Chang SX (2004) Substrate type, temperature, and moisture content affect gross and net N mineralization and nitrification rates in agroforestry systems. Biology and Fertility of Soils 39:269–279. DOI: 10.1111/j.1365-2745.2010.01789.x.
Zvereva EL, Kozlov MV (2011) Impacts of Industrial Polluters on Bryophytes: a Meta-analysis of Observational Studies. Water Air and Soil Pollution 218:573–586. DOI: 10.1007/s11270-010-0669-5.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Jn., Shi, Fs., Xu, B. et al. Uptake and recovery of soil nitrogen by bryophytes and vascular plants in an alpine meadow. J. Mt. Sci. 11, 475–484 (2014). https://doi.org/10.1007/s11629-013-2707-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11629-013-2707-4