Abstract
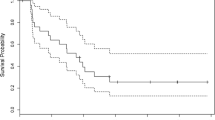
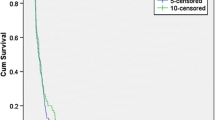
The treatment of recurrent glioblastoma (GBM) remains challenging notwithstanding the recent approval of bevacizumab for this indication. Bendamustine has a bifunctional mechanism of action including alkylation, penetrates the CNS and does not show cross resistance to other alkylator chemotherapies. In a single institution phase 2 trial, patients with recurrent GBM were treated with bendamustine (100 mg/m2/day administered intravenously for two consecutive days every 4 weeks). The primary study endpoint was 6-month progression free survival (PFS-6). An interim analysis for futility was conducted according to a Simon two-stage minimax design. Complete blood counts were obtained bimonthly, clinical evaluations and brain imaging every month for the first cycle and bimonthly thereafter. Treatment responses were based upon MacDonald criteria. Sixteen patients were enrolled (nine men; seven women), with a median age of 53 years (range 36–68) and a median Karnofsky performance status of 90 (range 70–100). Nine patients were treated at first relapse and seven at second relapse (five patients were bevacizumab failures). A total of 25 cycles of bendamustine were administered with a median of 1 (range 1–6). Bendamustine-related toxicity was seen in eight patients; lymphopenia in seven (5 grade 3; 2 Grade 4), thrombocytopenia in two (1 Grade 3; 1 Grade 4), and neutropenia in one (1 Grade 3). Fourteen patients have died due to disease progression, two patients are alive and on alternative therapies. Only one patient was progression-free at 6 months, triggering the stopping rule for futility. Bendamustine was reasonably well tolerated but failed to meet the study criteria for activity in adults with recurrent GBM.

Similar content being viewed by others
References
Adamson C, Kanu OO et al (2009) Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs 18(8):1061–1083
Brem H, Piantadosi S, Burger PC, Walker M, Selker R et al (1995) Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet 345:1008–1012
Fogh SE, Andrews DW, Glass J et al (2010) Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol 28(18):3048–3053
Carson KA, Grossman SA et al (2007) Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials. J Clin Oncol 25(18):2601–2606
Gerstner ER, Duda DG et al (2007) Antiangiogenic agents for the treatment of glioblastoma. Expert Opin Investig Drugs 16(12):1895–1908
Hou LCA, Veeravagu A et al (2006) Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus 20(4):E5
Yung WKA, Mechtler L, Gleason MJ (1991) Intravenous carboplatin for recurrent malignant gliomas: a phase II study. J Clin Oncol 9:860
Iwamoto FM, Fine HA (2010) Bevacizumab for malignant gliomas. Arch Neurol 67(3):285–288
Reardon DA, Fink KL, Mikkelsen T et al (2008) Randomized phase II study of cilengitide, an integrin-targeting arginine–glycine–aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol 26(34):5610–5617
Schmidt F, Fischer J, Herrlinger U et al (2006) PCV chemotherapy for recurrent glioblastoma. Neurology 66(4):587–589
Simpson L, Galanis E (2006) Recurrent glioblastoma multiforme: advances in treatment and promising drug candidates. Expert Rev Anticancer Ther 6(11):1593–1607
Wick W, Puduvalli VK, Chamberlain MC et al (2010) Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 28(7):1168–1174
Wick A, Pascher C, Wick W et al (2009) Rechallenge with temozolomide in patients with recurrent gliomas. J Neurol 256(5):734–741
See SJ, Levin VA, Yung A et al (2004) 13-cis-Retinoic acid in the treatment of recurrent glioblastoma multiforme. Neuro-Oncology 6:253–258
Chamberlain MC, Tsao-Wei D (2004) Recurrent glioblastoma multiforme: salvage therapy with cyclophosphamide. Cancer 100:1213–1220
Jaeckle KA, Hess KR, Yung A et al (2003) Phase II evaluation of temozolomide and 13-cis-retinoic acid for the treatment of recurrent and progressive malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol 21:2305–2311
Reardon DA, Wen PY, Desjardins A, Batchelor TT, Vredenburgh JJ (2008) Glioblastoma multiforme: an emerging paradigm of anti-VEGF therapy. Expert Opin Biol Ther 8(4):541–553
Yung WK, Albright RE, Olson J et al (2000) A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83(5):588–593
Coombs SE, Bischof M, Welzel T et al (2008) Radiochemotherapy with temozolomide as re-irradiation using high precision fractionated stereotactic radiotherapy (FSRT) in patients with recurrent gliomas. J Neurooncol 89(2):205–210
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Park JK, Hodges T, Arko L. et al (2009) Scale to predict survival after surgery for recurrent glioblastoma multiforme. Presented in part at the Annual Meeting of the Congress of Neurological Surgeons, New Orleans, 24–29 October 2009
Brada M, Stenning S, Gabe R et al (2010) Temozolomide versus procarbazine, lomustine and vincristine in recurrent high-grade glioma. J Clin Oncol 28:4601–4608
Reardon DA, Fink KL, Mikkelsen T et al (2008) Randomized phase II study of cilengitide, an integrin-targeting arginine–glycine–aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol 26:5610–5617
Nabors LB, Mikkelsen T, Rosenfeld SS et al (2007) Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol 25(13):1651–1657
Perry JR, Belanger K, Mason WP et al (2010) Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE Study. J Clin Oncol 28(12):2051–2057
Ballman KV, Buckner JC, Brown PD et al (2007) The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro-Oncology 9:29–38
Lamborn KR, Yung AWK, Chang SM et al (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. J Neurooncol 10:162–170
Wong ET, Hess KR, Gleason MJ et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572–2578
Bendamustine hydrochloride (CEP-18083) INVESTIGATOR’S BROCHURE, Cephalon Edition 2 Release Date: 4 March 2010
Keating MJ, Bach C, Yasothan U, Kirkpatrick P (2008) Bendamustine. Nat Rev Drug Discov 7(6):473–474
Leoni LM, Bailey B, Reifert J et al (2008) Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res 14(1):309–317
Köster W, Stamatis G, Heider A et al (2004) Carboplatin in combination with bendamustine in previously untreated patients with extensive-stage small cell lung cancer (SCLC). Clin Drug Investig 24(10):611–618
Köster W, Heider A, Niederle N et al (2007) Phase II trial with carboplatin and bendamustine in patients with extensive stage small-cell lung cancer. J Thorac Oncol 2(4):312–316
Eichbaum MH, Schuetz F, Khbeis T et al (2007) Weekly administration of bendamustine as salvage therapy in metastatic breast cancer: final results of a phase II study. Anticancer Drugs 18(8):963–968
Friedberg JW, Cohen P, Chen L et al (2008) Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin’s lymphoma: results from a phase II multicenter, single-agent study. J Clin Oncol 26(2):204–210
Lissitchkov T, Arnaudov G, Peytchev D, Merkle KH (2006) Phase-I/II study to evaluate dose limiting toxicity, maximum tolerated dose, and tolerability of bendamustine HCl in pre-treated patients with B-chronic lymphocytic leukaemia (Binet stages B and C) requiring therapy. J Cancer Res Clin Oncol 132(2):99–104
Friedberg JW, Cohen P, Chen L et al (2008) Bendamustine in patients with rituximab-refractory indolent and transformed non-Hodgkin’s lymphoma: results from a phase II multicenter, single-agent study. J Clin Oncol 26(2):204–210
Zulkowski K, Kath R, Semrau R, Merkle KH, Höffken K (2002) Regression of brain metastases from breast carcinoma after chemotherapy with bendamustine. J Cancer Res Clin Oncol 128:111–113
MacDonald DR, Cascino TL, Schold SC et al (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972
Cheson BD, Rummel M (2009) Bendamustine: rebirth of an old drug. J Clin Oncol 27(9):1492–1501
Acknowledgments
Carrie Graham, RN, CNP, Maciej Mrugala, MD and Alexander Spence, MD for contributing patients to the study; Brenda Kurland, PhD for statistical consultation and manuscript review, and Sharon Larsson for editorial assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chamberlain, M.C., Johnston, S.K. Salvage therapy with single agent bendamustine for recurrent glioblastoma. J Neurooncol 105, 523–530 (2011). https://doi.org/10.1007/s11060-011-0612-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0612-7