Abstract
Aquaculture is the fastest growing animal producing sector in the world and is expected to play an important role in global food supply. Along with this growth, concerns have been raised about the environmental effects of escapees and pollution, fish welfare, and consumer health as well as the use of marine resources for producing fish feed. In this paper we present some of the major challenges salmon farming is facing today. We discuss issues of relevance to how to ensure sustainability, by focusing on animal production systems, breeding approaches, sources for feed ingredients, and genetic engineering strategies. Other crucial issues such as animal welfare, environmental quality, and ethics are elaborated with regard to relevance for the sustainability of aquaculture. Additionally, we comment on socio-economic distributive implications by intellectual property rights (IPR) strategies on access to genetic material and traceability. To improve sustainability of salmon farming we suggest that there is a need for new approaches to guide research, for identification of ethical issues, and for engaging stakeholders in resolving these challenges.
Similar content being viewed by others
Introduction
The world population is growing, putting pressure on our natural resources. This is one of the challenges to sustainable development. A major issue is the production of animal protein, and there is an urgent need to find new protein sources. The aquaculture industry has the potential of contributing significantly to increasing the supply of seafood in the years ahead.
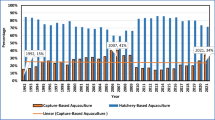
The Norwegian aquaculture industry has, since its breakthrough around 1970, developed from being a supplementary enterprise to a full-fledged industry. Economic incentives have, alongside this trend, led to rapid expansion of the production of carnivorous finfish species in marine aquaculture (Deutsch et al. 2007; Le Curieux–Belfond et al. 2009). At present, Norway is the world’s leading exporter of salmon and trout, with ca 50% of the world’s total salmon production in 2007 (740, 000 tons). In recent years, the export of Norwegian mariculture has increased 3–7% per year and is expected to continue to grow slowly in the years ahead.
During the past decade, the trend in the aquaculture industry has clearly been a concentration toward fewer, but larger fish farming enterprises. New technology is continuously being developed, and new species, such as Atlantic cod and Atlantic halibut, are being introduced as farmed species. Important barriers to increased growth in the industry are the losses due to diseases and parasites as well as access to sufficient economical feed ingredients, especially of marine fat and protein.
The growth of aquaculture in Norway has been accompanied by controversy between, e.g., environmental NGOs and fish farmers related both to potentially harmful internal effects on the fish themselves, namely animal welfare and health of the farmed salmon, and potentially harmful effects on the external environment. Concerns have also been raised about land-use change in coastal areas and the extensive use of marine resources for producing fish feed, impact on wild salmon by escapees from the farms, transmission of parasites and diseases between farms and to wild salmon, environmental pollution from the discharges, and socio-economic distributional effects of technological and regulatory developments in the aquaculture sector. Such concerns have also been expressed by consumers that request more environment friendly production practices (Frankic and Hershner 2003) and by national and international institutions (see for instance Holmenkollen guidelines for sustainable aquaculture (1998), FAO code of conduct on responsible fisheries (1995), the EU communication; “A strategy for the sustainable development of European aquaculture” (EC 2002), and Norwegian ministry of fisheries and costal affairs (2009); “Strategy for an environmental sustainable seafood industry”).
Sustainability is vaguely defined and it does not provide explicit directions as to what the values of sustainable development are and little guidance on how to set priorities. We therefore begin by discussing the concept of sustainability in relation to aquaculture practices. We use Norwegian salmon farming as a case, however, we want to emphasize that issues of relevance in Norwegian aquaculture are also of importance for aquaculture in other places. Section “Sustainability in Aquaculture: Animal Welfare and Health” is focused on the question of whether fish farming can be ethically justified from an animal welfare and health viewpoint. Because animal welfare is a vaguely defined concept, we focus and elaborate on aspects relating to feed and production methods (Sect. “Fish Farming Production Methods—Ethically Justified From an Animal Welfare Viewpoint”), and fish breeding (Sect. “Sustainability and Fish Breeding”). We draw on debates on animal welfare in agriculture and discuss what the implications may be for farmed fish. Subsequently, in section four we discuss the various effects that aquaculture may have on the external environment in terms of feed and resource use (Sect. “Sustainable Utilization of Natural Resources”), toxics, diseases, and genetic contamination relating to escaped fish (Sect. “Toxics, Diseases, and Genetic Contamination”), and issues relating to access and distribution of breeding material (Sect. “Regulating Access to Breeding Material in Aquaculture”). Finally, we make some suggestions about novel approaches to guide research, identify ethical issues and engage stakeholders in improving the sustainability of salmon farming.
Sustainable Development—The Concept and its Use in Aquaculture
The concept of sustainable development was defined by the Brundtland Commission as: “Sustainable development is development that meets the needs of the present without compromising the ability of future generations to meet their own needs” (WCED 1987). Later on, international Holmenkollen Guidelines (1999) for sustainable aquaculture have added and included the precautionary principle, the principles for environmental management inherent in the Rio Declaration of the UN Conference on Environment and Development, and the principles of Human Equity. The Rio Declaration takes into account the interdependence between biological, technological, socio-economic, and ethical aspects.
The concept of sustainability poses an improved tool for decision-making, as it brings together social, ecological, and economic considerations (Dovers et al. 1996). Aspects such as more equitable sharing of resources and improvement of ecology, e.g., environmental health and quality of life, are important issues of the sustainable framework. However, the Brundtland definition of sustainable development is broad and vague, which can be seen in the literature as different and contradictory interpretations of the normative values and the ethics behind sustainable development. The main contested values and practices of sustainable development may be values of sustainable development and how to set priorities (Kamara et al. 2006). How to achieve maintenance and preservation of nature and biodiversity versus a just society and versus economic development? Problems related to the practical implementation can be illustrated as follows: When people recognize that there is a problem, for instance disease in aquaculture that causes economic losses and reduces animal welfare or that the use of excessive marine feed need to be replaced with feed from other sources—people differ on which means should be used to solve this problem. These examples raise several issues; what kind of strategies should be employed to take all these considerations into the debate, who are the relevant stakeholders, and who is going to make the decision?
Sustainability in Aquaculture: Animal Welfare and Health
Promoting good husbandry practices and ensuring the welfare of farmed fish are well-established parts of the European Union policy for sustainable aquaculture development. However, there are often conflicts and trade-offs between short-term profit of the industry and demand for cheap animal products on one hand and animal welfare on the other, that the animals probably rarely gain from. Animal welfare has mostly been discussed in relation to research animals, land based animals for food production, and pets. Some of these issues will be highlighted before we move into implications of fish farming on animal welfare.
An important question with regard to animal husbandry is if it is morally legitimate to use animals merely as a resource or means to meet our needs, or if there are moral considerations that place restrictions on such an approach. Many difficult questions have arisen with regard to animals’ intrinsic value. Assuming that animals do have intrinsic value, all encroachments on their lives (by humans) become moral issues and demand carefully considered answers and actions. The Norwegian Animal Welfare Act of 2010, states that animals have an intrinsic value. This term contributes to clarifying that animal welfare must be prioritized irrespective of the value the animal may have for people, which also contributes to clarifying the animal’s status.
The word “welfare” is derived from well + fare, i.e., how well (or dignified) an animal “fares” (travels) through life. How well is an animal able to regulate its biological functions in relation to its environment? A function based definition of animal welfare is given by Broom (1986): “The welfare of an animal is its state as regards its attempts to cope with its environment.” Other definitions focus on an animal’s subjective experience or awareness of its condition (feeling based) and/or on whether it can lead a natural life (nature based). Hence, the term “animal welfare” applies to both the mental/emotional and physical health (from an objective standpoint) of the individual animal or the animal’s condition while trying to cope with its environment. The term also includes behavior, as well as physiological and immunological factors. In this context, health is defined more broadly than merely the absence of disease. It is also seen as a condition in which the body is resistant to negative environmental influences. An important basis for ensuring animal health is the animals’ well-being. Animal welfare is also more than just the absence of suffering. It also includes positive welfare, implying that denying an animal all positive experiences and stimuli is also an ethical problem with regard to animal protection. “Animal protection” is here seen as the protection of the mental/emotional and the physical health of each individual animal.
Most current animal ethicists use animals’ ability of sentience for ascribing direct moral considerations. Here, we support the claims of Lund et al. (2007) that fish welfare should be given serious moral considerations depending on their possession of the morally relevant similarities of sentience. The same authors reason further that fish are likely to be sentient and therefore deserve serious consideration. They also concluded from a simple risk analysis that the probability that the fish can feel pain is not negligible and that if they really do experience pain the consequence is great in terms of number of suffering animals. Hence, farmed fish should be given the benefit of doubt. Even from a more egoistic standpoint, we could argue for a fair treatment of animals. If we inflict suffering upon animals, we violate human dignity and may contribute to the development of a crueler society, as also indicated by Mahatma Gandhi.Footnote 1
Fish Farming Production Methods—Ethically Justified From an Animal Welfare Viewpoint?
Fish are cold-blooded animals, and thus especially vulnerable to extreme environmental conditions, such as temperature variations, currents, and algal blooms. In addition, farmed fish are also subject to handling, transport and chemical treatments (e.g., delousing). It has been shown that different fish species react differently to such conditions.
On the one hand, fish farms provide a protected environment for the fish—with few external enemies, plenty of feed and fresh, clean water. In addition, the fish are vaccinated against several diseases. On the other hand, however, fish populations in the net pens are very dense, resulting in a relatively high risk of disease and limited access to exercise and positive stimuli. Furthermore, the fish are extensively handled in connection with transport, vaccination, stripping (brood stock), and slaughter. Breeding and genetic engineering strategies can lead to permanent changes of the fish populations that may be of relevance for animal welfare.
When discussing whether modern fish farming can be ethically justified, two main issues must be considered:
-
What does modern fish farming actually imply for the fish?
-
With what should the conditions in a fish farm be compared to?
There is still insufficient knowledge about what factors are relevant for assessing the welfare of farmed fish. As a species, salmon actually show signs of having been domesticated in the course of their 30-year history as a farmed fish—showing less fear of humans and more frequently swimming in schools. Research on aspects of fish biology that are relevant for animal welfare issues, such as its sensory apparatus or its ability to sense fear, frustration, and pain, have not been given priority until recently. However, researchers have concluded that fish do have the physiological, anatomical, and biochemical prerequisites in their brain necessary for pain sensation (Chandroo et al. 2004, b; Sohlberg et al. 2004). Also, their behavior is typical of reactions to pain, discomfort, and fear.
Should the conditions in fish farming be compared to the fishes’ natural living conditions, to marine fisheries—or perhaps to the conditions in livestock husbandry on land? Many species obviously spend all or parts of their wild lives in densely packed schools (e.g., salmon at sea), whereas other species are much more territorial (e.g., river trout and salmon) in whole or parts of life. Farmed fish’ schooling behavior in relatively dense cages can be related to their wild relatives behavior during their sea phase. If the fish density in cages is too low, we may get more unfavorable territory protective and aggressive behavior as in the rivers. Wild fish can also suffer from diseases, get parasites, and even lack food for a while. However, when we keep fish in captivity, we become responsible for making sure that they do not suffer from disease or lack of food. We know little of fishes’ exercise requirements, or if they suffer when not being able to swim freely. Regarding mechanical strain in nature, one has to assume that a fish swimming against the current of a Norwegian river will have to endure somewhat of a “beating.” In traditional fisheries, using longlines, trawl, and fishing nets, as well as in game fishing, the fish are treated in ways that can inflict considerable pain. Even though such pain has, more or less consciously, been defined as “necessary” or “acceptable” suffering with regard to the Norwegian Animal Protection Act, one can not, however, conclude that this justifies poor welfare in the fish farming environment.
Fish Welfare Indicators
Recent findings strongly indicate that fish possess a degree of sentience (Chandroo et al. 2004, b; EFSA 2009; Sohlberg et al. 2004). Physiological, health, and behavioral status of individual fish have been used as indicators of compromised welfare (Huntingford et al. 2006). Behavior may serve as an early warning towards environmental changes (e.g., Beitinger 1990; Dawkins 2003) and is a general biomarker because behavioral traits are consequences of physiological and biochemical factors (Dawkins 2003). Thus behavior will give information regarding the animal’s health and welfare related to different causes. Furthermore, stress responses are often used as indicators of impaired welfare although physiological stress is not synonymous with suffering. Little is known about fish perception of its own welfare, and there is a lack of understanding how objectively measurable responses to challenges (physical damage and physiological and behavioral responses) are associated with subjective states of well-being or suffering (Huntingford et al. 2006). Chronic stress has a general immunosuppressive effect in fish (Weyts et al. 1999), and increased mortality due to pathogens (Pickering and Pottinger 1989) is the common outcome. More knowledge about diseases in fish, about the links between genetics, stress, immune function, and disease states and the relationship between health and welfare are needed. Hence, there is a need for methods and indicators and objective criteria for evaluating fish welfare.
The potential significance of brain serotonin (5-HT) for animal welfare is illustrated by its role in the pathophysiology of depression in mammals (e.g., Delgado et al. 1994; Porter et al. 2004). Previous studies in rainbow trout have revealed that delayed resumption of food intake after stress was associated with elevated brain stem concentrations of the main metabolite of 5-HT (Øverli et al. 2006). Elevated concentration of the serotonin metabolite, 5-HIAA and 5-HIAA/5-HT concentration ratio is a very general response to stress and adverse experiences in fishes, which occurs after, for instance social stress (Winberg et al. 1991; Øverli et al. 1999), predator exposure (Winberg et al. 1993), and confinement stress (Øverli et al. 2001). This neurochemical marker remains chronically elevated even after 4 weeks of stress (Winberg et al. 1991) and is also less sensitive to inadequate sampling procedures than plasma cortisol. It has been shown to reflect social organization and welfare in large groups of fish (Cubitt et al. 2008).
Data on fish physiology, biochemistry, and behavior are informative for fish welfare, but collecting them is time consuming, technically complex, and involves handling or killing fish in order to collect blood or tissue (Huntingford et al. 2006). Non-invasive methods exist (Ellis et al. 2004, Turner et al. 2003). However, these sometimes lack the precision of direct measurements made on individual fish. Examples of physical welfare indicators are changes in skin or eye color, which have been reported in a number of fish species. Eye color is an index of social stress/subordinate status in salmonids (O’Connor et al. 2000; Suter and Huntingford 2002). Abnormal swimming (Holm et al. 1998), unexpected loss of appetite (Huntingford et al. 2006), loss of body condition (Etscheidt and Manz 1992), and high frequency of injuries (e.g., dorsal fin injury caused by attacks from conspecifics) (Turnbull et al. 1998), are all signs of potentially impaired welfare in farmed fish.
Sustainability and Fish Breeding
In the Norwegian Animal Welfare Act (2010), it is stated that animal breeding shall encourage characteristics that give robust animals that function well and have good health. Reproduction, including gene technology, shall not be carried out in such a way that it:
-
a.
changes genes in such a way that they influence the animals’ physical or mental functions in a negative way, or continues pursuing such genes,
-
b.
reduces the animals’ ability to practice natural behavior, or
-
c.
stimulates general ethical reactions
Politically, it is currently not an option in Norway to base breeding on cloning or the use of transgenic farm animals or fish, since all farm animal associations and the Norwegian Seafood Federation clearly disapproved of such techniques. Nevertheless, gene technological methods are accepted when used as tools in laboratories to increase the efficiency of selecting breeding animals.
To test and rank families with regard to disease resistance, a group of fish from each family are infected with a disease agent, and the resulting fish mortality is recorded. These test fish presumably suffer, but their suffering is justified by the reasoning that a much larger number of fish can later be spared from similar suffering as a result of improved disease resistance. On the other hand, one could say that this is not very relevant to diseases for which the fish are vaccinated against anyway. However, such considerations cannot be applied to humans, and one may thus ask if it is ethically justifiable to do so with animals, including fish.
In the Report on animal welfare to the Norwegian Parliament, Stortinget (LMD 2008), specific challenges for fish breeding are discussed. Issues mentioned in the report include ensuring a selection of functionally healthy breeding fish, and making sure that the physical environment during incubation and hatching helps to reduce the frequency of side effects such as deformities and diseases.
The Norwegian Council for Animal Ethics (advisory body appointed by the Ministry of Agriculture and Food in collaboration with the Ministry of Fisheries and Coastal Affairs) made a statement about animal breeding in Norway and recommended as follows (Anonymous 2009):
-
1.
Efforts to make the fish even more able to sustain the current farm conditions while avoiding animal ethical dilemmas should be continued.
-
2.
The proportion of vegetable feeds must be adjusted to the current salmonids. Alternatively, one may consider breeding for developing salmonids that can utilize a bigger proportion of vegetable feeds without getting digestion disorders and diseases.
-
3.
The knowledge of basic physiological and genetic factors for health and welfare of farmed fish should be increased.
Due to concerns about using the limited marine fish resources for feeds, increased use of vegetable protein and oil is preferred and applied to some degree. However, as carnivorous species, salmonids have problems digesting diets with high proportion of vegetable ingredients, and may suffer from digestion diseases. Hence, the proportion of vegetable feeds must be balanced according to the physiology of the contemporary farmed fish. The fish ability to utilize and tolerate vegetable feeds may also be genetically improved by selection.
In contrast to the cooperatively organized breeding work for all other livestock species in Norway (with the exception of poultry, for which there are no national programs), salmon breeding enterprises are owned and operated by private breeding companies. These are either managed by specific limited companies (AquaGen and SalmoBreed) or as subsidiaries (or divisions) in a larger fish farming corporation (e.g., Marine Harvest). To start with, the Norwegian breeding material for farmed salmon was developed from broodstock from 40 Norwegian river populations. The very first salmon breeding program was cooperatively managed by Norsk Lakseavl, which in turn was owned by the Association of Norwegian Fish Farmers. After the liquidation of the Fish Farmers’ Sales Cooperative, the breeding program was taken over by private players in 1992, and organized as Aqua Gen AS.
These breeding companies conduct traditional breeding activities such as phenotype testing, sibling testing, and breeding-value appraisal, but some are also in production and sales of roe, fry, smolt, and consumer fish. Since fish breeding is carried out by private breeding companies, detailed breeding plans are not publicly available. However, the companies claim that their breeding programs are sound, taking inbreeding and multiple traits sufficiently into consideration. For example, salmon breeding focused to begin with mainly on performance, i.e., weight gain and maturity. Eventually, other traits such as fat contents, fat distribution, and meat color were included.
In the salmon industry, a few breeding populations have quickly become much more efficient than their non-improved and wild relatives, and thus, have come to dominate the market. Even though farmed fish are at an early stage of their domestication, with selection having been performed for only a few decades, a rapid selection response has already been documented for weight gain in several species. Due to fish reproduction and biology, it is possible to achieve such rapid progress through stringent selection. As a result, salmon farmers lack the same diversity of breeds that can be found in other livestock species—diversity necessary for crossbreeding and infusion of “new blood.” Crossing farmed fish with wild fish would result in considerable losses, due to the wild breeding stock’s inferior performance traits. The farmed fish populations are therefore highly vulnerable, increasing the need for restricting inbreeding and developing alternative strategies for the (re-)introduction of genetic variation. It is thus important to take the precautionary measures needed to avoid the occurrence of inbreeding and the negative development of traits as was observed in species with similar reproductive capacities, e.g., broilers and laying hens. To our knowledge, the family based salmon breeding programs in Norway endeavor to minimize inbreeding rate by maintaining a large number of families in each generation.
In fish farms and hatcheries, the fish adapt to the farm environment and get domesticated. With respect to fish welfare, it can be argued that changing animals to fit ethically acceptable production systems will reduce suffering. However, when applying more artificial selection, a reason for being careful and for monitoring selection responses is the considerable lack of biological understanding about physiology, behavioral needs, and fish welfare, in an industry that develop extremely fast. This applies to the direct response to those traits selected for, as well as to any correlated responses in other traits. However, the possible genetic changes in traits not recorded and selected for are challenging to monitor, as it may be resource demanding. On the other hand, unfavorable genetic changes may be costly for both fish breeders and farmers in the long term. For the fish welfare, they may certainly be critical as well.
Breeding Goals: Implications for Farm Animal Welfare
According to the Eurobarometer a common view in the European society is that sentient animals are the subjects of moral concern and that humans have a duty to consider their welfare because of their ability to suffer. This ethical standpoint regarding the moral status of sentient animals has been clearly expressed in several European surveys (e.g., Eurobarometer 58.0 2003).
Information about the welfare of the fish during production can be considered as an extrinsic product quality. Knowing that the fish had “a happy life” may contribute to the positive experience of a meal. Assuming the goal is profit maximization, economic values for other characteristics and traits such as productivity and growth can be derived using profit functions (e.g., Brascamp et al. 1985; Dekkers and Gibson 1998). However, defining breeding goals involving valuation of such intangibles as animal welfare in monetary terms is a challenge and has drawn increasing attention during the last decade (e.g., Torp Donner and Juga 1997; Olesen et al. 2000, 2006).
Animal welfare is a public good (McInerney 2004) and thus a characteristic of animal products that is not, or only partly, traded and valued in the marketplace (Randall 1987). Hence, market prices do not fully reflect the economic value people place on animal welfare (McInerney 2004). Studies indicate that Norwegian consumers don’t see animal welfare in food production as their responsibility (Kjørstad 2005). People direct this responsibility to the government and secondly to the producers and the retailers. Furthermore, consumers have very little knowledge about animal production and breeding (Ouédraogo 2003). Many consumers express concerns about farm animal welfare, but do not choose more expensive welfare labeled products (e.g., egg from free range layers). Lack of knowledge about animal production and lack of a feeling of responsibility may be reasons why there is a gap between attitudes and concerns about animal welfare, on the one hand, and buying practices, on the other. The above demonstrates that market prices of labeled and animal friendly products do not reflect the total economic value people place on animal welfare. To capture more of the total economic value of animal welfare, studies of people’s attitudes and willingness to pay (WTP) have been carried out (Bennett and Blaney 2002; Carlsson et al. 2007, Olesen et al. 2010). From a real choice (RC) market experiment, WTP for organic or Freedom food salmon was estimated at about 2 Euro extra per kg fillet compared to regular salmon (Olesen et al. 2010).
Breeding for Better Fish Health
At present diseases are causing economic problems and are affecting animal welfare issues. Diseases tend to multiply in farm environments and hence represent potential ecological threats both to the farmed fish itself and to the farm environment. Disease management through vaccine development and the use of antibiotics raises ethical issues with regard to animal welfare and environmental concerns and to the definition of welfare as natural living and behavior. Selection for increased disease resistance in fish is usually based on challenge tests carried out under controlled conditions. Challenge-tested fish cannot be used as parents for the next generation of elite salmon, meaning that selection cannot be applied directly on the breeding candidates. To circumvent this problem, geneticists have been searching for genes controlling the degree of resistance to different diseases. Markers for such genes would be ideal criteria for selection, since they could be applied directly on the animals without requiring challenge testing. Thus, the accuracy of selection could be increased while the need to sacrifice fish in challenge tests would be less.
Resistance against specific diseases has been emphasized in Norwegian salmon breeding since 1995. This is important for the fish themselves, producers and consumers alike. The Norwegian fish farming industry feels it should pioneer in this field, since Norway already has extensive experience from breeding for disease resistance in other livestock species, e.g., cattle. In 2007, Moen et al. (2009) identified markers for a gene that explains most (80%) of the genetic variation in resistance to infectious pancreatic necrosis (IPN) in both fry and post-smolts. Based on these findings, Aqua Gen has developed a tool for directly selecting IPN-resistant fish. This tool can, with very high accuracy, determine whether individual fish have zero, one, or two copies of the gene variant (allele) that give high resistance.
Salmon lice (Lepeophtheirus salmonis) presumably make the major health and welfare problem in the aquaculture industry today. Furthermore, it is also an ecological problem, since the lice multiply in fish farms, and then spread to the wild salmon population. Chemical treatment is used to combat the lice nowadays, but use of biological measures such as cleaner fish has increased lately due to more development of resistant lice to the chemical treatment. However, moderate genetic variation has been shown for resistance to the salmon louse, and thus it may be possible to reduce problems caused by lice through selective breeding programs (Kolstad et al. 2005). Breeding for disease resistance in Norwegian salmon and trout would increase the sustainability of the industry, and the know-how could be transferred to other aquaculture species.
Prospects for Genetic Improvement of Fish Welfare
Huntingford et al. (2006) list several factors in aquaculture that represent fish welfare challenges including aggressive interactions, handling and removal from water, diseases and permanent adverse physical states, and possibly increased levels of aggressiveness due to selection for fast growth.
Fish welfare was first indirectly taken into account in breeding programs for Atlantic salmon through the domestication process with adaptation to a life in captivity by selection for growth performance, where calm and less aggressive and presumably less stressed animals were selected due to their sufficient feed intake and faster growth. Secretion of growth hormone is reduced in fish during periods of stress (Pickering et al. 1991; Farbridge and Leatherland 1992), and may indicate a negative (but favorable) covariation between growth and stress. Appetite inhibition is a behavioral response to stress (Øverli et al. 1998), and is partly mediated by hormones involved in stress responses. Fevolden et al. (2002) reported significant and high heritability of the stress hormone, cortisol (0.50) in rainbow trout. Their findings gave support to better growth in a low cortisol responding trout selection line compared to a high responding line. It is further shown that high and low cortisol responding selection lines show divergent behavioral characteristics (Øverli et al. 2005). Work with salmonids shows that integrated behavioral and physiological mechanism that comprise the distinct “coping strategies” in mammals (Koolhaas et al. 1999) are also evident in fish, with heritable reactive and proactive traits demonstrated in rainbow trout (Øverli et al. 2005). This may indicate that selection for growth contributes to development of low responding and proactive animals that tend to be more dominant and aggressive. However, it has been shown in both salmon and cod that after a few generations of selection for growth and domestication in hatcheries and farms, we obtain calmer, less aggressive carnivorous fish. In a review of the effects of domestication on aggressive and schooling behavior in fish, Ruzzante (1994) concludes that domestication may strongly affect behavioral traits, but it is the intensity of the behavior rather than the behavioral pattern itself that is affected. Anyhow, more research is needed for a better understanding of the consequences of these processes.
Selection for high production efficiency in terrestrial animals is known to give undesirable effects in traits like health and reproduction (Rauw et al. 1998). Hence, selection for broader breeding goals including functional and welfare traits such as disease resistance have been applied in Nordic farm animal breeding. Since 1995, farmed salmon in Norway have been selected for resistance to diseases. Such selection for disease resistance will obviously reduce stress and suffering connected to diseases.
Apart from this, there is a lack of information on genetic variation of many welfare related traits such as behavior (e.g., aggression) and stress coping. In addition, information about their genetic covaration with other traits that are more or less related to fish welfare and selected for in the present breeding programs for salmon is lacking. Hence, we do not know whether there are unfavorably correlated responses in some fish welfare indicators, e.g., poorer ability to cope with stress, as a result of the present selection for productivity, fillet quality, and disease resistance.
It may become increasingly important to consider possibilities for more direct selection for fish welfare indicators in future breeding of salmon. Heritabilities of welfare traits for terrestrial farm animals have been estimated, but estimates for farmed fish are lacking. Differences between wild and captive fish populations have, however, been investigated and it is concluded that there are genetic differences between wild and captive populations of Atlantic salmon regarding survival and maturation (McGinnity et al. 1997) and behavior such as anti predator behavior (Petersson and Järvi 2006) and dominance (Metcalfe et al. 2003). Coho salmon artificially bred for four generations showed less aggressive behavior and a lower general activity than wild salmon (Fleming and Gross 1992). Hatchery reared salmonids showed a weaker antipredator response (Johnsson et al. 1996) and less physiological stress due to higher stocking densities (Mazur and Iwama 1993) when compared to the wild. As farmed fish adapt to the farm environment through generations, such domesticated fish will suffer less in the captive farm environment than wild fish. Hence, it can be argued that changing animals to better fit ethically acceptable production systems will reduce suffering and improve fish welfare.
Relevant fish welfare indicators or traits that currently can be taken into account in selective breeding are growth, survival (or mortality), social interactions/behavior (e.g., cannibalism for carnivorous species), and frequency of injuries (e.g., fin injuries) (Turnbull et al. 1998). For estimation of genetic parameters for such traits, some methodological issues also need to be studied. For example, different models can be applied to improve genetic analysis of survival time. Further, mixed model methodology for studying competitive or aggressive behavior affecting growth in fish exists (Muir and Schinkel 2002; Rutten et al. 2006), which may be particularly important when improving domestication of new cannibalistic species such as cod. These models may be applicable for analyzing genetic variation of aggression on other welfare traits such as fin injuries, deformities, survival, and feed intake. A main advantage of this method is that there is no need for monitoring and recording fish behavior for the sake of selection. However, for the sake of monitoring genetic changes to avoid other possible unfavorably correlated development in behavior, recording of behavior may still be needed at regular time intervals.
Non-Market Economic Values for Fish Welfare Traits
Low heritable traits such as health, welfare, and fertility might deteriorate even when these are included in the breeding goal. Such deterioration in traits important for animal welfare is obviously in conflict with animal welfare goals and may not be socially acceptable although it may be profitable for the individual farmer. Olesen et al. (2000) suggested weighing of each trait in the breeding goal by both their market and non-market values. By including non-market values in the breeding goal, such low heritable traits related to animal welfare can be given the appropriate social weight in order to avoid further deterioration due to intensive selection for production traits (Nielsen et al. 2005, 2006). As will be discussed more broadly in Sect. “Regulating Access to Breeding Material in Aquaculture,” patent systems tend to prefer what is more “easily” patented rather than what is “best” from a number of other perspectives (fish health, environment, socioeconomic, equity, and ethical). This raises the question of what strengthened systems for intellectual property rights (IPR), such as patents, may imply for breeding goals and the more general goal of stimulating sustainable aquaculture.
Sustainability in Aquaculture: Effects on the External Environment
Sustainable Utilization of Natural Resources
Thomson and Nardone (1999) discuss two different approaches to sustainable animal production: “resource sufficiency” and “functional integrity.” The first assumes that necessary production resources are available in the future, and are thus dependent on the rationalization of inputs and production. Functional integrity however, assumes that important parts of a production system (farm) are reproduced within the system over time in a way that is dependent on previous systems. The various parts can have both ecological and social limitations, and this approach is more in line with the definition of sustainability mentioned in Sect. “Sustainable Development—The Concept and its Use in Aquaculture”. In addition to environmental restrictions, it also includes economic, social, and cultural dimensions, as employed by the UN’s Food and Agriculture Organization (FAO 1992):
“Sustainable development is the management and conservation of the natural resources base, and the orientation of technological and institutional change in such a manner as to ensure the attainment and continued satisfaction of human needs for present and future generations. Such sustainable development in the agriculture, forestry and fisheries sectors conserves land, water, plant and animal genetic resources, is environmentally non-degrading, technically appropriate, economically viable and socially acceptable.” If “animal needs” was added to “human needs” in this definition, the sustainability concept may be much improved with respect to the moral standard of the society.
In Norway, there is a general high level of awareness about the importance of environmental and social factors for agriculture and aquaculture. One could thus say that in, e.g., Norway the term “sustainable food production” is a multi-dimensional concept, as, e.g., the agriculture policy includes a broad range of objectives such as maintaining rural settlement, protecting breeds at risk, preventing soil degradation, and giving priority to animal welfare (The Norwegian Government 2005). This is also reflected in present regulations of fish farms and production (Act of Aquaculture with associated regulations for salmon farmingFootnote 2). For instance, the production of roe, smolt, and consumer fish is regulated by concession rules and limits. The maximum annual biomass allowed per concession is currently fixed at 325 and 780 tonnes in fresh water and sea water, respectively. Consumer fish production is regulated by several laws and regulations, which specify such parameters as fish farm size and management. In addition, facilities are assessed with regard to their environmental effects based on information about current and standardized environmental analyses including seabed conditions. While the objective of the recent and current policy seems to fulfill many criteria of sustainable aquaculture, it still remains to see that the necessary measures are implemented for succeeding to meet its goals.
The fishing industry is mainly based on the utilization of wild fish, with an annual global catch of about 60 million tons of consumer fish per year. This figure has been rather stable for several years, and it is not expected that the catch can be increased substantially in the future. Salmon feed has largely been based on fish meal (about 40–60%) and fish oil (about 20–30%) from wild marine fish such as anchovies, pilchards, mackerel, herring, and blue whiting. Hence, a future constraint for expansion of salmon aquaculture is to find good substitutes for fish and fish oil as resources in feed. Furthermore, the use of fish for producing feed has caused critical questions concerning the environmental sustainability of using marine resources in salmon feed production, as it takes more than one unit of wild fish as feed input to produce one unit of farmed salmon (Naylor et al. 2000). Hence, the future of salmon aquaculture depends on the adoption of alternative sustainable feed resources in order to reduce the need for fish meal and fish oil. Potential new resources in salmon feed include species from lower trophic levels, by-products and by-catch from fisheries and aquaculture, animal by-products, plants, genetically modified (GM) plants, and products from microorganisms and GM microorganisms. Especially, the replacement of traditional fish feed with GM feed, as GM plants with improved nutritional constituents and modified oil has potential for reducing the need of feed sources from the marine environment.
In a recent study by Gillund and Myhr (2010), perspectives on alternative feed resources for salmon fish were identified among stakeholders in Norwegian aquaculture. In this study, the participants defined a broad range of appraisal criteria concerning health and welfare issues, economical issues, environmental issues, and knowledge and social issues that illustrates that finding sustainable alternative feed resources is not an easy task. For instance, there are divergent opinions on the benefits, concerns, and uncertainties when evaluating GM plants, and species from lower trophic levels. When assessing environmental issues, the participants in this study expressed opposing views with regard to whether current knowledge about species from lower trophic levels is sufficient to define sustainable levels of harvest. Moreover, the sustainability of plant production in industrial agriculture, and particularly the cultivation of GM plants, was contested among the participants. Hence, the authors argue that there is a need of further research on the suitability of alternative feed resources for farmed salmon and also that stakeholders should be involved to identify perspectives on the different alternative resources.
The new possibilities raised by genetic engineering has made it possible to develop transgenic fishes, genetically modified (GM) plants as edible vaccines and GM feed, GM and DNA vaccines, all of which may offer a technological solution to some of the problems aquaculture is struggling with at present. On the other hand, such introduction involves ecological risk; (i) transgenic fish may breed with wild fish, (ii) GM feed could be spread to the aquatic environment and consumed by other marine organisms, and (iii) horizontal gene transfer may occur from DNA in feed or vaccines to the recipient genome or by feces to the environment (Myhr and Dalmo 2005). These potential implications raise important questions; what type of applications should be permitted, and where do we draw the line? Should gene transfers between species be banned? This discussion also relates to the issue of patenting these new products developed by genetic engineering, genetically modified organisms (GMO), and products derived from these (more in Sect. “Regulating Access to Breeding Material in Aquaculture”).
There are often trade-offs between efficiency and considerations for health and the environment. Under consideration of the precautionary principle, attention is given to the identification of risk, scientific uncertainty, and ignorance, and it involves transparent and inclusive decision-making processes (Kriebel et al. 2001). In the case of valuable technology that enables the improvement of human health or even the saving of lives, such as the use of animals as bioreactors in the production of medicines, a certain risk of adverse effects can be accepted. However, if the expected benefits are insignificant, e.g., a slight drop in food prices, or if the risk of adverse side effects is relatively large, this could justify the limited use of such technology on the basis of ethical considerations. A common viewpoint is thus that the use (or non-use) of genetic engineering as such is not a relevant question, but rather the trade off between any benefits and the adverse effects of possible side effects where significant emphasis is put on the inherent uncertainties.
Some of these issues are regulated in Norway by the Gene Technology Act of 1993, which cites contribution to sustainable development and societal utility as criteria for accepting any GM product or produce. In a recent report requested by the Norwegian Directorate of Nature Management (Myhr and Rosendal 2009), it was found that there is very little information both in the literature and in marketing applications of GMO received by EU authorities from GMO developers on how GMOs contribute to any of these criteria. Furthermore, they found that most risk assessment and management of GMOs lack an acknowledgment of the relationship between short term concerns for human health and more long term concerns for environmental consequences. Accordingly, the authors emphasized the need for research on how GMOs affect sustainability in Norway as well as in countries that Norway may import GMOs and GM based products from.
Toxics, Diseases, and Genetic Contamination
The contents of persistent organic environmental pollutants (POPs) such as dioxins and PCBs in farmed salmon are among the greatest challenges with regard to its food safety. Hites et al. (2004) showed that farmed salmon contained more organochlorine contaminants compared to wild salmon, and that European raised salmon had higher contaminant loads than North and South American salmon. They also raised concerns about the possible health risks associated (increased number of cancer deaths) to consumption of farmed salmon due to the concerted effect of all POPs, although the levels for each pollutant were below the maximum recommended levels. Fish feed was also analyzed and suggested as the main source of POPs in the salmon. However, the benefit from salmon in human diets is debated, and several argue that the cardiovascular benefits outweigh possible harmful effects (Tuomisto et al. 2004). Anyhow, reducing the amount of pollutants in the fish feeds may reduce the health risks of salmon consumption considerably. Efficient methods for removing POPs from fish oil have been developed (Breivik and Thorstad 2002, 2005). The reason that the fish oil used in the fish feed has not been purified is probably due to higher costs. However, more use of vegetable oil and oils from South America in salmon feeds has probably reduced the POPs in Norwegian salmon considerably in recent years. High levels of vegetable oils will, however, reduce the level of the favorable (healthy) n-3 poly unsaturated fatty acids, i.e., EPA and DHA.
Anyhow, recent trials have shown that salmon fed feeds containing decontaminated and purified fish oil are healthier and have a firmer fillet, and that the feed is also utilized better (Hægermark 2009). This should definitely be included in the evaluation of whether or not it is profitable to use purified fish oil in salmon feeds. On commission for Pronova BioPharma, salmon trials through the smolt phase to the full grown salmon that had reached slaughter size were carried out with feed containing purified and not purified fish oil. Growth, health effects, and fillet quality of the fish and possible effects of feed containing purified or not purified fish oil was studied. The trials showed a trend of better growth and feed utilization among salmon that consumed the feed containing purified oil. Furthermore, the salmon that received the feed with purified oil appeared to better tackle the handling stress during the slaughtering process—measured as a delayed pH reduction after killing. Other stress markers showed the same tendencies. Another important advantage by cleaning fish oils is that by doing this, the feed and aquaculture industry may contribute to removing POPs from the natural food chains, and prevent further accumulation and harmful effects in wild organisms.
Public opinion is an important factor for further development of aquaculture. As a result, the aquaculture industry has become very aware of its importance and has drastically reduced its use of antibiotics. In 1987, the administration of antibiotics in Norwegian fish farming reached its maximum of 49 tonnes. The administration to all Norwegian farmed fish in 2007 was 648 kg, which makes a reduction of ca 800 kg, or 56% from 2006 (Directorate of Fisheries 2009). While nearly 0.9 kg antibiotics was used per tonne of slaughtered salmon and trout in 1987, the administration is now below 1 g per tonne.
Escapees; Ethical Issues and Implications for Biodiversity
There has been widespread debate about, and considerable fear of the possible negative effects of escaped farmed fish on wild populations. Genetic interaction between escaped farmed fish and wild populations has been proven (Crozier 1993; Clifford et al. 1998; McGinnity et al. 2003; Hindar and Jonsson 1995). Environmental and genetic differences between farmed fish and wild populations include fitness traits such as survival, body shape, growth, competitiveness, and reproduction. Escapes of farmed salmon have been significantly reduced; from 1.6–2 million (2–3% of the total farmed fish production) in the late 1980s, to 0.4 million (0.1% of the total farmed fish production) in 2002. The recent years, the number of escapees has increased again up to 0.7 million (Jonsson et al. 2006). It is reported that the farmed salmon make on average 11–35% of the total number of broodfish in the rivers. The escaped farmed salmon compete with the wild salmon for food, habitats and spawning grounds, they spread parasites and diseases and through mating, they change the genetics of the wild populations. Salmon louse is considered the most serious disease threat to the wild salmon in Norway. There are regulations for registration of number of salmon lice on farmed salmon as well as for delousing (e.g., with chemicals such as benzoate and pyrethroids) to keep the level of salmon louse larvae low in the period that the wild salmon smolt move out to the sea (spring and early summer). Hence, maximum number per fish is 0.5 female salmon lice from January to June. In some areas they synchronize the delousing in different farms to improve the efficiency of delousing.
Since 2003, salmon farming has been banned in several large fjords in Norway to protect important, threatened salmon populations (Directorate of Fisheries 2010). Today, a total of 52 national salmon river systems and 29 national salmon fjords are protected. There are several reasons for this protection: salmon has an important value as a cultural icon in Norwegian society, wild-salmon fisheries is considered important for recreation and for income, and it is considered important to protect the genetic diversity of the wild salmon populations. Not least, Norway has a special management responsibility for wild Atlantic salmon: About 50% of this species is found within Norwegian jurisdiction, and Norway is obliged under the Convention on Biological Diversity to take measures for its conservation and sustainable use.
Genetic Impact of Escaped Farmed Salmon on Wild Populations
The conservation of the genetic diversity of wild salmon populations is important, both to maintain the wild salmon populations and as a source of potentially valuable diversity for future breeding work. However, it is difficult to totally prevent farmed fish from escaping, so there will always be some interaction with wild fish. Concerning genetic interactions and implications, Verspoor et al. (2007) concluded that farm escapes can have significant direct and indirect negative impacts on wild populations by reducing productivity and mean fitness of wild populations by competitive, disease, and parasite interactions. Effective containment and considered location of farms, involving epidemiological zones and vaccination programs to control disease and parasites, as well as maximizing domestication of farm strains, are the best ways to ensure avoidance of direct and indirect genetic impacts.
Genetic variation in the farmed fish populations can provide a basis for natural selection to counteract the loss of fitness in the affected wild populations. Breeding programs based on many families and broad breeding goals will help to maintain such genetic variation and avoid the loss and fixation of alleles. The use of broodstock from several local populations in the establishment of a breeding program’s base population also reduces the risk of introducing new alleles in local populations via escaped farmed fish.
Tufto (2001) modeled the effects of migration on the population size and evolution of a quantitative trait. He concluded that genetic difference between wild and farmed fish populations was especially important for the effect of farmed fish on wild fish populations. A moderate genetic gain thus reduces the probability of a negative effect on the wild fish populations. Of course, this is less desirable for the fish farmers. Alternatively, one could imagine that large, rapid genetic improvements would be more favorable, since this would reduce the fitness of the farmed fish to such a degree that a genetic contribution from these to the next generation of wild fish could be avoided.
Neutral alleles without any effect on the fitness of wild fish can be lost in the farmed fish populations as a result of random drift. The loss of alleles can then be transferred to the wild populations through the long-term impact from escaped farmed fish, as discussed by Bentsen and Thodesen (2005). Alleles that are neutral today may, however, play an important role for fitness in the future. An option would thus be to cross the farmed fish with individuals from wild populations to reduce the risk of losing such alleles. Crossing different breeding populations is another, and perhaps easier and less costly strategy for the prevention of such allele losses.
The use of sterile, triploid farmed fish has also been suggested as a way to prevent the genetic impact on wild fish populations. However, this strategy is costly in Atlantic salmon, due to slower growth. It may provoke ethical concerns among consumers and there may be unknown ecological effects of unchecked growth, because triploid fish do not reach sexual maturity.
Genetic Impact of Escaped Transgenic Farmed Salmon on Wild Populations
Improvement of transgenic techniques and functional genomics has opened up vast possibilities for the development of transgenic fish. These possibilities include enhancement of the quality of cultured stocks by improving growth rate and cost effectiveness, increasing resistance to disease and stress, and creating new or different products through alteration of the fish genome (Melamed et al. 2002). In particular, the ability to manipulate growth rates through the introduction of growth hormone (GH) genes has been applied frequently in transgenic fish research (FAO 2003), and this has been most successful in species such as Atlantic salmon, coho salmon, Nile tilapia, and hybrid tilapia. By the application of genetic engineering, an increase of growth rate in juvenile salmon has been achieved that was 4–11 times the rate of non-transgenic controls (Devlin et al. 1994, 2004). Devlin et al. (2001) found that although the transgenic trout grew much faster than non-transgenic wild sibling controls (achieving a 17.3-fold difference in weight by 14 months post-fertilization), introducing the growth-hormone construct into a domestic strain did not cause further growth enhancement. Research by AquaBounty (subsidiary of A/F Protein Inc.) has resulted in the AquAdvantage, a transgenic Atlantic salmon that contains a gene construct composed of the regulatory elements of an ocean pout antifreeze protein gene controlling expression of a chinook salmon GH gene. The company is seeking approval for commercial use of this transgenic fish, and the application has been under evaluation in almost 10 years by the US Food and Drug Administration (Niiler 2000).
There are obvious economic advantages arising from application of genetic engineering to fish; however, transgenic fish also involve potential ecological hazards and ethical dilemmas to society. There are numerous examples of the substantial problems that can be caused by the introduction of plant and animal species to new habitats. It must also be concluded from previous studies that we only have very limited knowledge about possible negative side effects. Often, we lack both the creativity and the know-how to ask the necessary critical questions, which would enable us to examine the potential risks beforehand. The use of transgenic fish is thus a gigantic experiment with nature.
During commercial aquaculture, transgenic fish will certainly escape into the environment. The environmental impact may depend on the number of escaped fish, their phenotypic characteristics (related to ability for reproduction and survival over time), and the aquatic biodiversity present in the receiving ecosystem (Kapuscinski and Brister 2001). At present, there are three dominant hypotheses with regard to potential ecological effects by release or escape of transgenic fish (Muir and Howard 2001, 2002; Pew Initiative on Food and Biotechnology 2003):
-
The Trojan gene hypothesis; if there is a trade-off of fitness costs and benefits due to expression of a transgene, the impact of introgression of the transgene into the population will depend upon the net impact on fitness.
-
The Purge hypothesis; if the transgenic fish has lower fitness than the wild-type conspecifics, hybridization will cause the transgene(s) to be purged from the species through natural selection, and the wild stocks may persist.
-
The Spread hypotheses; if the fitness of the transgenic fish is equal to or exceeds that of its wild-type conspecifics, hybridization will cause the transgene(s) to enter into the wild population and will result in a loss of genetic diversity.
The question with regard to whether the release or escape of transgenic fish will result in ecological impacts needs to be scientifically substantiated. Interestingly, in order to minimize the risk of transgenic fish breeding with wild populations after accidental release or escape, genetic engineering has been applied for producing either sterile fish or fish where reproductive activity can be specifically down-regulated with inducible promoters (Melamed et al. 2002). Such promoters can be turned on through exposure to specific chemicals or metals. These characteristics would be of value, allowing both optimal growth and controlled reproduction of the transgenic fish, while ensuring that any escaped fish would be unable to breed.
Regulating Access to Breeding Material in Aquaculture
The rapidly increasing value and interest in marine genetic resources, coupled with their vulnerability due to loss of biodiversity and challenges for obtaining a fair distribution of the benefits make the issues of access and legal protection of breeding material particularly relevant. Breeding companies, marine bioprospecting institutions, and investors in genetic engineering need protection of their improved genetic material to assure revenues from innovation and investment. At the same time, these same actors as well as fish farmers also need access to marine genetic resources for food production and further innovation and development (Rosendal et al. 2006; Olesen et al. 2007). For prolific animals such as poultry or fish, pirate breeding of just a few individuals can be extremely efficient and lead to considerable losses for the breeding companies. Protective strategies such as hybrid breeding have often been used, e.g., in pig and poultry breeding, whereas royalty agreements have been made between breeding companies and buyers for pigs and various fish species. Combined with continuous upgrading of the genetic material through selective breeding, this also seems to be the preferred strategy among fish breeders (Olesen et al. 2007).
Olesen et al. (2007) found that the predominant view among Norwegian salmon breeders was that the sector needs a balance between access to breeding material and protection of proprietary innovations in fish breeding. Furthermore, there was an emerging realization that the value of improved breeding material is underestimated, leaving the farmers to reap most of the added value from fish breeding and farming. Consequently, there is an emerging interest in finding some way of capturing the value of the improved stocks among the fish breeders. Rosendal et al. (2006) presented the rationale for ensuring access to and protecting genetic resources in aquaculture, and discussed different options for protecting genetic improvements with respect to investment returns and possibilities for further innovation, access, and genetic diversity. Few other empirical studies have been conducted pertaining to the links between innovation and intellectual property rights in farmed animals and aquaculture (Greer and Harvey 2004). The issue has only recently been included in the working agenda of the Commission on Genetic Resources in the Food and Agriculture Organisation of the UN (FAO).
Access to genetic resources pertaining to aquaculture is vital for the future development of this sector, which is becoming increasingly important in Norway along with other parts of the world, not least with a high potential for increasing food security in developing countries. For instance, carp farming makes a major proportion of the global aquaculture, and is important as a relatively inexpensive protein source. Breeding efforts have, for example, resulted in genetic improvement of about 20% faster growth per generation of farmed Rohu carp in India (Anonymous 2004). A major challenge in shrimp and fish farming is diseases, which cause severe losses and risks. Access to resistant breeding material will therefore be crucial for both shrimp and fish farmers and breeders in all parts of the world. Access to medicines and vaccines being developed for fish and shrimp diseases may be even more important to the actors in this sector. There are large economic stakes involved and a wide array of choices concerning legal and biological protection of genetic improvements.
Currently, relevant types of legal regimes at national and international levels are under development and meanwhile domestic and external activities take place in an unclear legal situation (Tvedt 2005). However, researchers and smaller research institutions may find other options than patents more feasible and rewarding for their activities and innovations because they lack the institutional resources to pursue and enforce patent rights. Currently, fish farmers have equal access to the genetic improvements, and the breeding system is able to supply enough spawn to cover the entire industry’s demand. The biggest breeding company (AquaGen) is now more or less foreign-owned, following the German EW group’s purchase of 52% of AquaGen. The effects of patents are uncertain as the structural development in the aquaculture sector is increasingly turning to privatization and market consolidations (fewer and larger market actors). Biologically, the aquaculture sector is less adjusted to the use of patents compared to plants, but this may change with the application of gene technology, which may bolster patent strategies for competitive reasons. The real value in breeding programs lies, however, in continued upgrading and improvement and patents are not useful for this.Footnote 3 In effect, distributional issues, such as the objective of securing access for the poor market actors, become increasingly topical also in aquaculture. In addition, the patent debate involves the question of effects on new breeding goals, such as animal welfare and environmental aspects (see Sect. “Sustainability and Fish Breeding”). This has clear parallels to the situation and debate characterizing the privatized pharmaceutical R&D sector, where developments of new vaccines and medicines increasingly turn to cater mostly for the needs of those who can afford to pay the market price (Sandberg 2009).
Genetic Engineering as Drivers for Patenting
There has been a strong policy to stimulate patenting in research and development projects, an activity that is also stimulated by genetic engineering applied within pharmacology and medicine as well as genetically modified organisms (Rosendal et al. 2006:403). The fast growth of aquaculture has also stimulated research into salmon genomics and the corresponding problems due to diseases have triggered a massive effort into development of efficient vaccines where, for instance, use of GM and DNA vaccines is one important strategy.
Increased knowledge about the genome of the fish species will increase the applicability of the patent system for protecting the commercial use of such knowledge. Genetic engineering may reduce the barriers to patenting inventions, but has so far not been much applied in animal breeding in spite of high expectations for a long period (Olesen et al. 2007). Genetic modification strategies used for development of transgenic fish will facilitate protection through patenting in the same way as other genes, and it may be easier to enforce patent rights of transgenic fish by identification of the unique gene construct that is transmitted and that will not be present in other fish. This may be a driving force towards permitting production of transgenic fish also in Europe, where a restrictive GMO policy has been practiced. Noiville (1999) focused on the risks of the patent law in the traditional breeding and also the problem with broad patents. On a similar note, Rye (2000) has stressed the problem of lack of legal mechanisms for sharing the benefits between a patent holder and the breeder or owner of the fish population, which constitutes the origin of the patented gene or animal. Moreover, if there are many patents in one field of technology, it may become difficult and costly for new inventors to obtain licenses from all patent holders. Such practical and monetary obstacles may hinder the development of new inventions in a technical field.
Implications of Demands for Traceability
Consumers’ increasing demand for traceability of food products has resulted in an improved documentation and infrastructure of the information about the production through the whole production chain. Soon, only marginal improvements on the logistics and documentation are needed to fulfill the requirements for implementing mandatory pedigree certificate as described by Rosendal et al. (2006). A trace system may then be used to ensure that the breeder receives royalties according to the material transfer agreement for the use of their brood stock. It may be relatively easy to establish such a system on the national level, but an international system will be rather challenging to initiate and enforce, and hence this needs further considerations and analysis.
New Approaches and Recommendations to the Identified Challenges
Olesen et al. (2000) reviewed impact of characteristics of future sustainable production systems and development, and emphasized the need for long-term biologically, ecologically, and sociologically sound breeding goals, because animal breeding (and hence production) determined only by short term market forces leads to unwanted side effects. Implementation of both market and non-market values was recommended to allow for breeding programs that contribute to sustainable production systems. Important prerequisites for sustainable production are appropriate governmental policies, awareness of our way of thinking, and a more communal worldview informed by a subjective epistemology and holistic ontology (Olesen et al. 2000). Agriculture policies in Norway and EU have many good objectives covering a range of criteria required for sustainable production as well as many regulations to fulfill many of these. Emphasizing values other than short term economic profit may be challenging for a vulnerable industry in a competitive global market and that is subject to biological and ecological factors and operating with small margins. Hence, it still remains to be seen whether the requested priorities of non-market values of, e.g., animal ethics and long-term goals of managing environmental goods and natural resources, are given in aquaculture regulations and production. This will depend on both the Governmental institutions as well as the industry that will have to increase their efforts to succeed on sustainable aquaculture.
In the EU communication “A strategy for the sustainable development of European aquaculture,” it is stated that scientists should “recognize the responsibility to develop and make available the best technology, in particular for the efficient use of the resources and for avoiding harm to the environment” (European Commission 2002). Accordingly, scientists are challenged to acknowledge the potential adverse effects of science and technology as well as their products to the environment. Such a challenge involves application of a precautionary approach that includes initiation of targeted studies of specific hazards and that ensures that preventive measures are incorporated into a research and policy agenda that encourage broad and long-term thinking, and that initiates integrative research to identify benefits, risks as well as uncertainties. Furthermore, the complexity of aquaculture necessitate that different scientific disciplines need to be involved as compatible providers of information and models for studying the problem or the system. Interdisciplinary approaches will bring redundancies into the process and entail a challenge to the different disciplines to understand and be respectful of other interpretations, methods, and models, but will evolve the scientific understanding and improve awareness of sustainability. The societal aspects and ethical issues involved entails that deliberative approaches are also needed to be employed to help in identifying what is considered as sustainable aquaculture.
Such approaches can open for discussions about, e.g., how animal welfare is affected by different practices and can identify perspectives on the resources for feed and the use of new technologies. This may help to get more broad views that would help to address the wide array of implications as well as the significance of uncertainty for adverse effects. One such approach is upstream engagement that has been developed and applied with the purpose of avoiding future conflicts over life-science research and its applications. Upstream engagement holds potential for broad discussion to identify social, ethical, and legal implications and can help to reduce the time lag, distance, and power asymmetry between “upstream” and “downstream” innovation processes (Felt and Wynne 2007). Hence, upstream engagement represents an approach that will require dialogue and framework building, with multi-stakeholder involvement of scope. Although developed for life-sciences technology, this approach can also be applicable for identification of indicators and perspectives of sustainability of aquaculture. Furthermore, this approach leads to a shared collective responsibility. The other benefit is that such an approach will identify perspectives on longer-term developments, as well as institutionalization of practices. Hence, such approaches can be used to open up debates on what is considered as sustainable animal production, breeding, resources for feed, and the goals that genetic engineering should be applied to meet, as well as what is considered as acceptable implications on, e.g., animal welfare and the use of IPR in salmon farming.
Notes
Quote of Mahatma Gandhi: “The greatness of a nation and its moral progress can be judged by the way its animals are treated.”
Forskrift om tillatelse til akvakultur for laks, ørret og regnbueørret (Laksetildelingsforskriften). http://www.lovdata.no/for/sf/fi/fi-20041222-1798.html.
This is a preliminary finding from the project “Stimulating sustainable innovation in aquaculture” (Project Number: 187970, funded by the Research Council of Norway).
References
Anonymous. (2004). Final report on the Indo-Norwegian collaborative projects (56 pp.). Bhubaneswar: Central Institute of Freshwater Aquaculture (CIFA).
Anonymous. (2009). Dagens husdyravl i et etisk perspektiv. (only available in Norwegian) http://www.radetfordyreetikk.no/2009/06/dagens-husdyravl-i-et-etisk-perspektiv/. Accessed 29 April 2010.
Beitinger, T. L. (1990). Behavioral reactions for the assessment of stress in fishes. Journal of Great Lakes Research, 16, 495–528.
Bennett, R. M., & Blaney, R. (2002). Social consensus, moral intensity and willingness to pay to address a farm animal welfare issue. Journal of Economic Psychology, 23, 501–520.
Bentsen, H. B., & Thodesen, J. (2005). Genetic interactions between farmed and wild fish, with examples from the Atlantic salmon case in Norway. In T. Gjedrem (Ed.), Selection and breeding programs in aquaculture (pp. 319–334). Dordrecht, The Netherlands: Springer.
Brascamp, E. W., Smith, C., & Guy, D. R. (1985). Derivation of economic weights from profit equations. Animal Production, 40, 175–180.
Breivik, H., Thorstad, O. (2002). WO/2004/007654—a process for decreasing environmental pollutants in an oil or a fat, a volatile environmental pollutants decreasing working fluid, a health supplement, and an animal feed product. http://www.wipo.int/pctdb/en/wo.jsp?IA=IB2003002827&wo=2004007654.
Breivik, H., & Thorstad, O. (2005). Removal of organic environmental pollutants from fish oil by short-path distillation. Lipid Technology, 17(3), 55–58.
Broom, D. M. (1986). Indicators of poor welfare. British Veterinary Journal, 142, 524–526.
Carlsson, F., Frykblom, P., & Lagerkvist, C. J. (2007). Consumer willingness to pay for farm animal welfare: Mobile abattoirs versus transportation to slaughter. European Review of Agriculture Economics, 34, 321–344.
Chandroo, K. P., Duncan, I. J. H., Moccia, R. D., et al. (2004a). Can fish suffer? Perspectives on sentience, pain, fear and stress. Applied Animal Behavior Science, 86, 225–250.
Chandroo, K. P., Yue, S., Moccia, R. D., et al. (2004b). An evaluation of current perspectives on consciousness and pain in fishes. Fish and Fisheries, 5, 281–295.
Clifford, S. L., McGinnity, P., & Ferguson, A. (1998). Genetic changes in Atlantic salmon (Salmo salar) populations of northwest Irish rivers resulting from escapes of adult farm salmon. Canadian Journal of Fisheries and Aquatic Science, 55, 358–363.
Crozier, W. (1993). Evidence for genetic interaction between escaped farmed salmon and wild Atlantic salmon (Salmo salar L.) in a northern Irish river. Aquaculture, 113, 19–29.
Cubitt, K. F., Winberg, S., Huntingford, F. A., Kadri, S., Crampton, V. O., & Øverli, Ø. (2008). Social hierarchies, growth and brain serotonin metabolism in Atlantic salmon (Salmo salar) kept under commercial rearing conditions. Physiology & behavior, 94, 529–535.
Dawkins, M. S. (2003). Behavior as a tool in the assessment of animal welfare. Zoology, 106, 383–387.
Dekkers, J. C. M., & Gibson, J. P. (1998). Applying breeding objectives to dairy cattle improvements. Journal of Dairy Science, 81, 9–35.
Delgado, P. L., Price, L. H., Miller, H. L., Salomon, R. M., Aghajanian, G. K., Heninger, G. R., et al. (1994). Serotonin and the neurobiology of depression—effects of tryptophan depletion in drug-free depressed-patients. Archives of General Psychology, 51, 865–874.
Deutsch, L., Gräslund, S., Folke, C., Troell, M., Huitric, M., Kautsky, N., et al. (2007). Feeding aquaculture growth through globalization: Exploitation of marine ecosystems for fishmeal. Global Environmental Change, 17, 238–249.
Devlin, R. H., Biagi, C. A., & Yesaki, T. Y. (2004). Growth, viability, and genetic characteristics of GH transgenic coho salmon strains. Aquaculture, 236, 607–632.
Devlin, R. H., Biagi, C. A., Yesaki, T. Y., Smailus, D. E., & Byatt, J. C. (2001). Growth of domesticated transgenic fish. A growth-hormone transgene boosts the size of wild but not domesticated trout. Nature, 409, 781–782.
Devlin, R. H., Yesaki, T. Y., Biagi, C. A., Donaldson, E. M., Swanson, P., & Chan, W. K. (1994). Extraordinary salmon growth. Nature, 371, 209–210.
Directorate of Fisheries (2010) Marine protected areas. http://www.fiskeridir.no/english/fisheries/marine-protected-areas. Accessed 29 April 2010.
Directorate of Fisheries (Fiskeridirektoratet) (2009) Nøkkeltall fra norsk havbruksnæring År 2008. (only available in Norwegian). http://www.fiskeridir.no/statistikk/akvakultur/statistiske-publikasjoner/noekkeltall-fra-norsk-havbruksnaering. Accessed 29 April 2010.
Dovers, S. R., Norton, T. W., & Handmer, J. W. (1996). Uncertainty, ecology, sustainability and policy. Biodiversity Conservation, 5, 1143–1167.
EFSA (2009). Scientific opinion of the panel on animal health and welfare on a request from European Commission on general approach to fish welfare and to the concept of sentience in fish. The EFSA Journal, 954, 1–26. http://www.efsa.europa.eu/en/scdocs/scdoc/954.htm. Accessed 29 April 2010.
Ellis, T., James, J. D., Stewart, C., & Scott, A. P. (2004). A non-invasive stress assay based upon measurement of free cortisol released into the water by rainbow trout. Journal of Fish Biology, 65, 1233–1252.
Etscheidt, J., & Manz, D. (1992). Suswasseraquaristik und tierarzliche Praxis. Teil 2: Untersuchungen zur artgerechten Haltung von Zierfuschen. Tierärztliche Praxis, 20, 221–226.
Eurobarometer (2003) 58.0, 2nd edition, 21 March. A report to the Directorate General for the project ‘Life sciences in European society’ QLG7-CT-1999-00286.
European Commission (2002). A strategy for the sustainable development of European aquaculture. European Commission, Brussels. http://ec.europa.eu/fisheries/cfp/aquaculture_processing/aquaculture/strategy_en.htm. Accessed 29 April 2010.
FAO. (1992). Sustainable development and environment: FAO policies and actions, Stockholm 1972-Rio 1992. Rome: FAO.
FAO (1995). Code of conduct on responsible fisheries. Fisheries and Aquaculture Department, Food and Agricultural Organisation of the United Nations, Rome. ftp://ftp.fao.org/docrep/fao/005/v9878e/v9878e00.pdf. Accessed 29 April 2009.
FAO (2003). Genetically modified organisms and aquaculture. FAO Fisheries Circular No. 989, Rome.
Farbridge, K. J., Leatherland, J. F. (1992). Plasma growth-hormone levels in fed and fasted rainbow-trout (Oncorhynchus-mykiss) are decreased following handling stress. Fish Physiology and Biochemistry, 10, 67–73.
Felt, U.,& Wynne, B. (2007). Taking European Knowledge Society Seriously. European Communities, Directorate-General for Research Science, Economy and Society. http://ec.europa.eu/research/science-society/document_library/pdf_06/european-knowledge-society_en.pdf. 95 pp. Accessed 10 July 2009.
Fevolden, S. E., Røed, K. H., & Fjalestad, K. T. (2002). Selection response of cortisol and lysozyme in rainbow trout and correlation to growth. Aquaculture, 205, 61–75.
Fleming, I. A., & Gross, M. R. (1992). Reproductive-behavior of hatchery and wild coho salmon (oncorhynchus-kisutch)—does it differ. Aquaculture, 103, 101–121.
Frankic, A., & Hershner, C. (2003). Sustainable aquaculture: Developing the promise of aquaculture. Aquaculture International, 11, 517–530.
Gillund, F.,& Myhr, A. I. (2010) Perspectives on Salmon feed—A deliberative assessment of several alternative feed resources. Journal of Agriculture and Environmental Ethics, Available online 28 Jan.
Greer, D., & Harvey, B. (2004). Blue genes. sharing and conserving the world’s aquatic genetic resources (p. 231). London: Earthscan.
Hægermark, W. Aa. (2009). Several advantages with purified feed oil. http://www.nofima.no/marin/en/nyhet/2009/08/several-advantages-with-purified-feed-oil. Accessed 29 April 2010.
Hindar, K., & Jonsson, B. (1995). Impacts of aquaculture and hatcheries on wild fish. In D. P. Philipp, J. M. Epifanio, J. E. Marsden, & Claussen (Eds.), Protection of aquatic biodiversity. Proceedings of the World Fisheries Congress, Theme 3 (pp. 70–87). New Delhi: Oxford and IBH Publishing.
Hites, R. A., Foran, J. A., Carpenter, D. O., Coreen Hamilton, M., Knuth, B. A., & Schwager, S. J. (2004). Global assessment of organic contaminants in farmed salmon. Science, 303, 226–229.
Holm, J. C., Tuene, S., Fosseidengen, J. E. (1998). Halibut behavior as a means of asssessing suitability of ongrowth systems. Annual Science Conference, ICES. Casias, Portugal 16–19 Sept.
Holmenkollen Guidelines (1999). Holmekollen Guidelines for sustainable aquaculture 1997. In: N. Svennevig, H. Reinertsen, M. New (Eds.) Sustainable Aquaculture (pp. 343–347) Proceedings of the Second International Symposium on Sustainable Aquaculture: Food for the future? November 2–5, 1997, Oslo, Norway: AA. Balkema, Rotterdam/Brookfield.
Huntingford, F. A., Adams, C., Braithwaite, V. A., Kadri, S., Pottinger, T. G., Sandøe, P., et al. (2006). Current issues in fish welfare. Journal of Fish Biology, 68, 332–372.
Johnsson, J. I., Petersson, E., Joensson, E., Bjoernsson, B. T., & Jaervi, T. (1996). Domestication and growth hormone alter antipredator behavior and growth patterns in juvenile brown trout, Salmo trutta. Canadian Journal of Fisheries and Aquatic Sciences, 53, 1546–1554.
Jonsson, B., Boxaspen, K., Fiske P., Gjerde, B., Poppe, T., Wennevik, V. (2006). Interaksjoner mellom lakseoppdrett og villaks: Oppdatering av kunnskapen etter NOU 1999:9. Kunnskapsserien for laks og vannmiljø 2. pp. 80.
Kamara, M., Coff, C., & Wynne, B. (2006). GMOs and sustainability: Contested visions, routes and drivers. Copenhagen: Report prepared for the Danish Council of Ethics.
Kapuscinski, A. R., & Brister, D. J. (2001). Genetic impacts of aquaculture. In K. D. Black (Ed.), Environmental impacts of aquaculture (pp. 128–153). Sheffield, UK: Sheffield Academic press.
Kjørstad, I. (2005). Welfare quality WP1.1 Consumers literature review country report Norway (p. 60). Oslo Norway: SIFO, National Institute for Consumer Research.
Kolstad, K., Heuch, P. A., Gjerde, B., Gjedrem, T., & Salte, R. (2005). Genetic variation in resistance of Atlantic salmon (Salmo salar) to the salmon louse Lepeophtheirus salmonis. Aquaculture, 247, 145–151.
Koolhaas, J. M., Korte, S. M., De Boer, S. F., Van Der Vegt, B. J., Van Reenen, C. G., Hopster, H., et al. (1999). Coping styles in animals: Current status in behavior and stress-physiology. Neuroscience and Biobehavioral Reviews, 23, 925–935.
Kriebel, D., Tickner, J., Epstein, P., Lemons, J., Levins, R., Loechler, E. L., et al. (2001). The precautionary principle in environmental science. Environmental Health Perspectives, 109, 871–876.
Le Curieux–Belfond, O., Vandelac, L., Caron, J., & Seralini, G. E. (2009). Factors to consider before production and commercialization of aquatic genetically modified organisms: The case of transgenic salmon. Environmental Science and Policy, 12, 170–189.
LMD (2008) St.meld. nr. 12 (2002-2003) Om dyrehold og dyrevelferd. (only available in Norwegian). http://www.regjeringen.no/nb/dep/lmd/dok/regpubl/stmeld/20022003/Stmeld-nr-12-2002-2003-.html?id=196533. Accessed 28 April 2010.
Lund, V., Mejdell, C., Röcklinsberg, H., Anthony, R., & Håstein, T. (2007). Expanding the moral circle: Farmed fish as objects of moral concern. Diseases of Aquatic Organisms, 75, 109–118.
Mazur, C. F., & Iwama, G. K. (1993). Effect of handling and stocking density on hematocrit, plasma-cortisol, and survival in wild and hatchery-reared chinook salmon (oncorhynchus-tshawytscha). Aquaculture, 112, 291–299.
McGinnity, P., Prodöhl, P., Ferguson, A., Hynes, R., Maoiléidigh, N., Baker, N., et al. (2003). Fitness reduction and potential extinction of wild populations of Atlantic salmon, Salmo salar, as a result of interactions with escaped farm salmon. Proceedings of the Royal Society: Biological Sciences, 270, 2443–2450.
McGinnity, P., Stone, C., Taggart, J. B., Cooke, D., Cotter, D., Hynes, R., et al. (1997). Genetic impact of escaped farmed Atlantic salmon (Salmo salar L.) on native populations: use of DNA profiling to assess freshwater performance of wild, farmed, and hybrid progeny in a natural river environment. ICES Journal of Marine Science, 54, 998–1008.
McInerney, J. (2004). Animal Welfare, economics and policy. Report on a study for Defra. pp. 68.
Melamed, P., Gong, Z., Fletcher, G., & Hew, C. L. (2002). The potential impact of modern biotechnology on fish aquaculture. Aquaculture, 204, 255–269.
Metcalfe, N. B., Valdimarsson, S. K., & Morgan, I. J. (2003). The relative roles of domestication, rearing environment prior residence and body size in deciding territorial contests between hatchery and wild juvenile salmon. Journal of Applied Ecology, 40, 535–544.
Moen, T., Baranski, M., Sonesson, A. K., & Kjøglum, S. (2009). Confirmation and fine-mapping of a major QTL for resistance to infectious pancreatic necrosis in Atlantic salmon (Salmo salar): Population-level associations between markers and trait. BMC Genomics, 10, 368.
Muir, W. M., & Howard, R. D. (2001). Fitness component and ecological risk of transgenic release; A model using Japanese medaka (Oryzius latipes). American Naturalist, 159, 1–16.
Muir, W. M., & Howard, R. D. (2002). Assessment of possible ecological risks and hazards of transgenic fish with implications for other sexually reproductive organisms. Transgenic Research, 11, 101–114.
Muir, W. M., Schinkel, A. (2002). Incorporation of competitive effects in breeding programs to improve productivity and animal well being. Proceedings from the 7th World Congress on genetics applied to livestock production. Montpellier, France, communication no. 14–07.
Myhr, A. I., & Dalmo, R. A. (2005). Introduction of genetic engineering in aquaculture: Ecological and ethical implications for science and governance. Aquaculture, 250, 542–554.
Myhr, A. I., Rosendal, G. K. (2009). GMO Assessment in Norway as Compared to EU Procedures: Societal Utility and Sustainable Development. DN-Utredning 2009-2 http://www.dirnat.no/content.ap?thisId=582 Accessed 15 Jan 2009.
Naylor, R. L., Goldberg, R. J., Primavera, J. H., Kautsky, N., Beveridge, M. C. M., Clay, J., et al. (2000). Effect of aquaculture on world fish supplies. Nature, 405, 1017–1024.
Nielsen, H. M., Christensen, L. G., & Groen, A. F. (2005). Derivation of sustainable breeding goals for dairy cattle using selection index theory. Journal of Dairy Science, 88, 1882–1890.
Nielsen, H. M., Christensen, L. G., & Ødegård, J. (2006). A method to define breeding goals for sustainable dairy cattle production. Journal of Dairy Science, 89, 3615–3625.
Niiler, E. (2000). FOA researchers consider first transgenic fish. Nature Biotechnology, 18, 143.
Noiville, C., (1999). Farm animal breeding and the law. In: A.M. Neeteson-van Nieuwenhoven (Ed.), The future developments in farm animal breeding and reproduction and their ethical, legal and consumer implications (pp 91–100). Report EC-ELSA project 4th Framwork Programme for RTD Nov 1999.
Norwegian Ministry of Fisheries and Costal Affairs (2009). Strategi for en miljømessig bærekraftig havbruksnæring. (Only available in Norwegian). www.regjeringen.no/…strategier…/strategi-for-en-miljomessig-barekraftig-.html. Accessed 10 July 2009.
O’Connor, K. I., Metcalf, N. B., & Taylor, A. C. (2000). The stability of standard metabolic rate during a period of food deprivation in juvenile Atlantic salmon. Journal of Fish Biology, 57, 41–51.
Olesen, I., Alfnes, F., Røra, M., Navrud, S., & Kolstad, K. (2010). Eliciting consumers’ willingness to pay for organic and welfare labelled salon by a non-hypothetical choice experiment. Livestock Science, 127, 218–226.
Olesen, I., Groen, A. F., & Gjerde, B. (2000). Definition of animal breeding goals for sustainable production systems. Journal of Animal Science, 78, 570–582.
Olesen, I., Navrud, S., Kolstad, K. (2006). Economic values of animal welfare in breeding goals. Invited paper. Proceedings from the 8th World Congress on genetics applied to livestock production. August 13–18, 2006, Belo Horizonte, MG, Brasil CD-ROM: 538-1710.pdf.
Olesen, I., Rosendal, G. K., Walløe Tvedt, M., Bryde, M, Bentsen, H. B. (2007). Access to and protection of aquaculture genetic resources—strategies and regulations. Aquaculture, 272S1, 47–61.
Ouédraogo, A. (2003). Symbolic goods in the market place. Public perceptions of farm animal breeding and reproduction in France and the United Kingdom. In A. E. Liinamo, A. M. Neeteson-van Nieuwenhoven (Eds). SEFABAR Sustainable European farm animal Breeding and reproduction. Final workshop, Rome, 4 sep 2003. pp. 36–46.
Øverli, Ø., Harris, C. A., & Winberg, S. (1999). Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in Rainbow trout. Brain, Behavior and Evolution, 54, 263–275.
Øverli, Ø., Pottinger, T. G., Carrick, T. R., Øverli, E., & Winberg, S. (2001). Brain monoaminergic activity in rainbow trout selected for high and low stress responsiveness. Brain, Behavior and Evolution, 57, 214–224.
Øverli, Ø., Sørensen, C., & Nilsson, G. E. (2006). Behavioral indicators of stress-coping style in rainbow trout: Do males and females react differently to novelty? Physiology & Behavior, 87, 506–512.
Øverli, Ø., Winberg, S., Damsgård, B., & Jobling, M. (1998). Food intake and spontaneous swimming activity in Arctic char (Salvelinus alpinus): Role of brain serotonergic activity and social interactions. Canadian Journal of Zoology, 76, 1366–1370.
Øverli, Ø., Winberg, S., & Pottinger, T. G. (2005). Behavioral and neuroendocrine correlates of selection for stress responsiveness in rainbow trout—a review. Integrative and Comparative Biology, 45, 463–474.
Petersson, E., & Järvi, T. (2006). Anti-predator response in wild and sea-ranched brown trout and their crosses. Aquaculture, 253, 218–228.
Pew Initiative on Food and Biotechnology (2003). Future fish: issues in science and regulation of transgenic fish. Washington, Pew Initiative on Food and Biotechnology. http://www.pewtrusts.org/uploadedFiles/wwwpewtrustsorg/Reports/Food_and_Biotechnology/hhs_biotech_011403.pdf Accessed 5 July 2006.
Pickering, A. D., & Pottinger, T. G. (1989). Stress responses and disease resistance in salmonid fish—effects of chronic elevation of plasma-cortisol. Fish Physiology and Biochemistry, 7, 253–258.
Pickering, A. D., Pottinger, T. G., Sumpter, J. P., Carragher, J. F., & Le Bail, P. Y. (1991). Effects of acute and chronic stress on the levels of circulating growth hormone in the rainbow trout, Oncorhynchus mykiss. General and Comparative Endocrinology, 83, 86–93.
Porter, R. J., Gallagher, P., Watson, S., & Young, A. H. (2004). Corticosteroid-serotonin interactions in depression: A review of the human evidence. Psychopharmacology, 173, 1–17.
Randall, A. (1987). Resource economics. New York: John Wiley & Son.
Rauw, W. M., Kanis, E., Noordhuizen-Stassen, E. N., & Grommers, F. J. (1998). Undesirable side effects of selection for high production efficiency in farm animals. A review. Livestock Production Science, 56, 15–33.
Rosendal, G. K., Olesen, I., Bentsen, H. B., Walløe Tvedt, M., & Bryde, M. (2006). Access and legal protection of aquaculture genetic resources—Norwegian Perspectives. The Journal of World Intellectual Property, 9, 392–412.
Rutten, M. J. M., Bovenhuis, H., Komen, H., Bijma, P. (2006). Mixed model methodology to infer whether aggression increases due to selection on growth in aquaculture species. Proceedings from the 8th World Congress on genetics applied to livestock production August 13–18, 2006, Belo Horizonte, MG, Brasil. CD-ROM: 432-2143.pdf.
Ruzzante, D. E. (1994). Domestication effects on aggressive and schooling behavior in fish. Aquaculture, 120, 1–24.
Rye, M. (2000). Bipatenter–mulige konsekvenser for genetisk foredlingsarbeid i akvakultur. In S. Rogne & O. J. Borge (Eds.), Biopatenter og EUs patentdirektiv. (only available in Norwegian). Rapport møte 29 September 2000 (pp. 26–27). Oslo: Bioteknologinemnda.
Sandberg, K. I. (2009) International Health Policies. The Institutional Dimension of Global Child Immunization. PhD thesis. Faculty of Medicine, University of Oslo.
Sohlberg, S., Mejdell, C., Ranheim, B., & Søli, N. E. (2004). Oppfatter fisk smerte, frykt og ubehag?: En litteraturgjennomgang. (only available in Norwegian). Norsk Veterinærtidsskrift, 116, 429–438.
Suter, H. C., & Huntingford, F. A. (2002). Eye colour in juvenile Atlantic salmon: Effects of social status, aggression and foraging success. Journal of Fish Biology, 61, 606–614.
The Norwegian Government (2005). Plattform for regjeringssamarbeidet mellom Arbeiderpartiet, Sosialistisk Venstreparti og Senterpartiet 2005–09. 73 sider. (only available in Norwegian). http://www.regjeringen.no/nb/dep/smk/dok/rapporter_planer/rapporter/2005/soria-moria-erklaringen.html.
Thompson, P. B., & Nardone, A. (1999). Sustainable livestock production: Methodological and ethical challenges. Production Science, 61, 111–119.
Torp Donner, H., & Juga, J. (1997). Sustainability-a challenge to animal breeding. Agricultural and Food Science in Finland, 6, 229–239.
Tufto, J. (2001). Effects of releasing maladapted individuals: A demographic-evolutionary model. American Naturalist, 158, 331–340.
Tuomisto, J. T., Tuomisto, J., Tainio, M., & Niittynen, M. (2004). Risk-benefit analysis of eating farmed salmon. Science, 305, 476.
Turnbull, J. F., Adams, C. E., Richards, R. H., & Robertson, D. A. (1998). Attack site and resultant damage during aggressive encounters in Atlantic salmon (Salmo salar L.) parr. Aquaculture, 159, 345–353.
Turner, J. W., Nemeth, R., & Rogers, C. (2003). Measurement of fecal glucocorticoids in parrotfishes to assess stress. General and Comparative Endocrinology, 133, 341–352.
Tvedt, Morten Walløe. (2005). Har noen eksklusive tinglige rettigheter til genetiske ressurser i Norge? (The Legal Situation Regarding Exclusive Rights to Genetic Resources in Norway). Retfærd, Nordisk juridisk tidsskrift, 109, 70–90. In Norwegian.
Verspoor, E., Olesen, I., Bentsen, H. B., Glover, K., Mcginnity, P., Norris, A. (2007). Genetic effects of domestication, culture and breeding of fish and shellfish, and their impacts on wild populations. Atlantic salmon-Salmo salar. In T. Svåsand, D. Crosetti, E. García-Vázquez, E. Verspoor (Eds). Genetic impact of aquaculture activities on native populations. Genimpact final scientific report (EU contract RICA-CT-2005-022802). p. 23–31.
WCED. (1987). Our common future. Oxford: Oxford University Press.
Weyts, F. A. A., Cohen, N., Flik, G., & Verburgvan Kemenade, B. M. L. (1999). Interactions between the immune system and the hypothalamo-pituitary-interrenal axis in fish. Fish and Shellfish Immunology, 9, 1–20.
Winberg, S., Myrberg, A. A., & Nilsson, G. E. (1993). Predator exposure alters brain serotonin metabolism in bicolor damselfish. Neuroreport, 4, 399–402.
Winberg, S., Nilsson, G. E., & Olsén, K. H. (1991). Social rank and brain levels of monoamines and monoamine metabolites in Arctic charr, Salvelinus alpinus (L). Journal of Comparative Physiolology, A168, 241–246.
Acknowledgments
Financial support from Research Council of Norway through the project “Stimulating sustainable innovation in aquaculture” (Project number 187970/S10) for this paper is acknowledged, as well as the funding by Nordforsk of the Nordic Network on Agriculture and Food Ethics. The authors will also thank Dr. Karl Shearer for valuable discussions and comments to the paper and the anonymous reviewer for their comments on an earlier version of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olesen, I., Myhr, A.I. & Rosendal, G.K. Sustainable Aquaculture: Are We Getting There? Ethical Perspectives on Salmon Farming. J Agric Environ Ethics 24, 381–408 (2011). https://doi.org/10.1007/s10806-010-9269-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10806-010-9269-z