Abstract
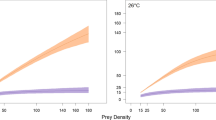
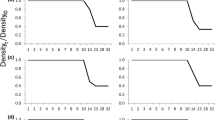
Population density can have profound, often negative effects on fitness-related traits and population dynamics, and density dependence is of central importance to many prominent ecological and evolutionary hypotheses. Here, we used experimental manipulations of food, population density, and water conditioning to characterize the mechanisms underlying reproductive density-dependence in Potamopyrgus antipodarum. This New Zealand freshwater snail is a prominent model system for invasion biology, ecotoxicology, and the maintenance of sexual reproduction. We demonstrated that a primary source of negative density-dependence is food limitation, but surprisingly, we found that P. antipodarum reproductive output was much higher in high density versus low-density conditions when food was adequate. We then used manipulations of water environment to demonstrate that these positive effects of high density are likely caused by a waterborne substance produced by P. antipodarum. Altogether, these results indicate that there are strong and complex connections between food availability, density, and reproductive output in this important model system that could influence the dynamics of invasive populations, the costs and benefits of sex, and the approaches used for ecotoxicology studies.


Similar content being viewed by others
References
Alonso A, Castro-Díez P (2012) The exotic aquatic mud snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca): state of the art of a worldwide invasion. Aquat Sci 74:375–383
Baur B, Baur A (1990) Experimental evidence for intra- and interspecific competition in two species of rock-dwelling snails. J Anim Ecol 59:301–315
Bonenfant C, Gaillard JM, Coulson T, Festa-Bianche M, Loison A, Garel M, Egil Loe L, Balanchard P, Pettorelli N, Owen-Smith N, Du Toit J, Duncan P (2009) Empirical evidence of density-dependence in populations of large herbivores. Adv Ecol Res 41:313–357
Brenneis V, Sih A, DeRivera CE (2010) Coexistence in the intertidal: interactions between the non-indigenous New Zealand mud snail Potamopyrgus antipodarum and the native estuarine isopod Gnorimosphaeroma insulare. Oikos 119:1755–1764
Brook BW, Bradshaw CJA (2006) Strength of evidence for density dependence in abundance time series of 1,198 species. Ecology 87:1445–1451
Cameron RA, Carter MA (1979) Intra- and interspecific effects of population density on growth and activity in some helicid land snails (Gastropoda: Pulmonata). J Anim Ecol 48:237–246
Chamaillé-Jammes S, Fritz H, Valeix M, Murindagomo F, Clobert J (2007) Resource variability, aggregation and direct density dependence in an open context: the local regulation of an African elephant population. J Anim Ecol 77:135–144
Cope NJ, Winterbourn MJ (2004) Competitive interactions between two successful molluscan invaders of freshwaters: an experimental study. Aquat Ecol 38:83–91
Cross WF, Benke AC (2002) Intra-and interspecific competition among coexisting lotic snails. Oikos 96:251–264
Dan N, Bailey SER (1982) Growth, mortality and feeding rates of the snail Helix aspersa at different population densities in the laboratory, and the depression of activity of helicid snails by other individuals or their mucus. J Moll Stud 48:257–265
Dillon RT Jr (2000) The ecology of freshwater molluscs. Cambridge University Press, Cambridge
Doncaster CP, Pound GE, Cox SJ (2000) The ecological cost of sex. Nature 404:281–285
Dorgelo J (1987) Density fluctuations in populations (1982–1986) and biological observations of Potamopyrgus jenkinsi in two trophically differing lakes. Hydrobiol Bull 21:95–110
Duft M, Schmitt C, Bachmann J, Brandelik C, Schulte-Oehlmann U, Oehlmann J (2007) Prosobranch snails as test organisms for the assessment of endocrine active chemicals- an overview and a guideline proposal for a reproduction test with the freshwater mudsnail Potamopyrgus antipodarum. Ecotoxicology 16:169–182
Dybdahl MF, Lively CM (1995) Diverse, endemic and polyphyletic clones in mixed populations of a freshwater snail. J Evol Biol 8:385–398
Fowler CW (1987) A review of density dependence in populations of large mammals. Curr Mammal 1:401–441
Gust M, Buronfosse T, André C, Mons R, Gagné F, Garric J (2011) Is exposure temperature a confounding factor for the assessment of reproductive parameters of New Zealand mudsnails Potamopyrgus antipodarum (Gray)? Aquat Toxicol 101:396–404
Haase M (2003) Clinal variation in shell morphology of the freshwater gastropod Potamopyrgus antipodarum along two hill-country streams in New Zealand. J Roy Soc New Zealand 33:549
Jacobsen R, Forbes VE (1997) Clonal variation in life-history traits and feeding rates in the gastropod, Potamopyrgus antipodarum: performance across a salinity gradient. Func Ecol 11:260–267
Jensen A, Forbes VE, Parker ED Jr (2001) Variation in cadmium uptake, feeding rate, and life-history effects in the gastropod Potamopyrgus antipodarum: linking toxicant effects on individuals to the population level. Environ Toxicol Chem 20:2503–2513
Jokela J, Lively CM, Dybdahl MF, Fox JA (1997) Evidence for a cost of sex in the freshwater snail Potamopyrgus antipodarum. Ecology 78:452–460
Jokela J, Lively CM, Taskinen J, Peters AD (1999) Effect of starvation on parasite-induced mortality in a freshwater snail (Potamopyrgus antipodarum). Oecologia 119:320–325
Kawata M (1993) Relative importance of direct and indirect interaction among individual snails. Res Popul Ecol 35:69–77
Kawata M, Ishigami H (1992) The growth of juvenile snails in water conditioned by snails of a different species. Oecologia 91:245–248
Krist AC, Lively CM (1998) Experimental exposure of juvenile snails (Potamopyrgus antipodarum) to infection by trematode larvae (Microphallus sp.): infectivity, fecundity compensation and growth. Oecologia 116:575–582
Krist AC, Jokela J, Wiehn J, Lively CM (2004) Effects of host condition on susceptibility to infection, parasite developmental rate, and parasite transmission in a snail-trematode interaction. J Evol Biol 17:33–40
Levy MG, Tunis M, Isserhoff H (1973) Population control in snails by natural inhibitors. Nature 241:65–66
Lively CM (1987) Evidence from a New Zealand snail for the maintenance of sex by parasitism. Nature 328:519–521
Lively CM (1992) Parthenogenesis in a freshwater snail: reproductive assurance versus parasitic release. Evolution 46:907–913
Lively CM (2009) The maintenance of sex: host—parasite coevolution with density-dependent virulence. J Evol Biol 22:2086–2093
McKenzie VJ, Hall WE, Guralnick RP (2013) New Zealand mudsnails (Potamopyrgus antipodarum) in Boulder Creek, Colorado: environmental factors associated with fecundity of a parthenogenic invader. Can J Zool 91:30–36
Negovetic S, Jokela J (2001) Life-history variation, phenotypic plasticity and maintenance of subpopulation structure in a freshwater snail. Ecology 82:2804–2815
Neiman M (2006) Embryo production in a parthenogenetic snail (Potamopyrgus antipodarum) is negatively affected by the presence of other parthenogens. Inv Biol 125:45–50
Neiman M, Lively CM (2004) Pleistocene glaciation is implicated in the phylogeographical structure of Potamopyrgus antipodarum, a New Zealand snail. Mol Ecol 13:3085–3098
Richards DC, Shinn DC (2004) Intraspecific competition and development of size structure in the invasive snail Potamopyrgus antipodarum (Gray, 1853). Am Malacolog Bull 19:33–37
Schreiber ESG, Glaister A, Quinn GP, Lake PS (1998) Life history and population dynamics of the exotic snail Potamopyrgus antipodarum (Prosobranchia: Hydrobiidae) in Lake Purrumbete, Victoria, Australia. Mar Freshwater Res 49:73–78
Sibly RM, Barker D, Denham M, Hone J, Pagel M (2005) On the regulation of populations of mammals, birds, fish and insects. Science 309:607–610
Song Y, Drossel B, Scheu S (2011) Tangled Bank dismissed too early. Oikos 120:1601–1607
Stange D, Oehlmann J (2012) Identification of oestrogen-responsive transcripts in Potamopyrgus antipodarum. J Moll Stud 78:337–342
Steele MA, Forrester GE (2005) Small-scale field experiments accurately scale up to predict density dependence in reef fish populations at large scales. Proc Nat Acad Sci USA 102:13513–13516
Thomas JD (1982) Chemical ecology of the snail hosts of schistosomiasis: snail—snail and snail-plant interactions. Malacologia 22:81–91
Thomas JD, Aram RH (1974) The chemical ecology of Biomphalaria glabrata (Say), the effects of media homotypically conditioned by adult snails on the growth of juveniles. J Exp Zool 190:329–339
Thomas JD, Goldsworthy GJ, Aram RH (1975) Studies on the chemical ecology of snails. The effects of chemical conditioning by adult snails on the growth of juvenile snails of the same species. J Anim Ecol 44:1–27
Tibbets TM, Krist AC, Hall RO Jr, Riley LA (2010) Phosphorus-mediated changes in life history traits of the invasive New Zealand mudsnail (Potamopyrgus antipodarum). Oecologia 163:549–559
Tobin PC, Berec L, Liebhold AM (2011) Exploiting Allee effects for managing biological invasions. Ecol Lett 14:615–624
Turchin P, Taylor AD (1992) Complex dynamics in ecological time series. Ecology 73:289–305
Vernon JG (1995) Low reproductive output of isolated, self-fertilizing snails: inbreeding depression or absence of social facilitation? Proc Roy Soc Lond B 259:131–136
Wagner M, Oelhmann J (2009) Endocrine disruptors in bottled mineral water: total estrogenic burden and migration from plastic bottles. Env Sci Pollut Res 16:278–286
Winterbourn MJ (1970) Population studies on the New Zealand freshwater gastropod Potamopyrgus antipodarum (Gray). Proc Malacolog Soc Lond 39:139–149
Woiwod IP, Hanski I (1992) Patterns of density dependence in moths and aphids. J Anim Ecol 61:619–629
Young JPW (1981) Sib competition can favour sex in two ways. J Theor Biol 88:755–756
Zeng Z, Nowierski RM, Taper ML, Dennis B, Kemp WP (1998) Complex population dynamics in the real world: modeling the influence of time-varying parameters and time lags. Ecology 79:2193–2209
Acknowledgments
We thank Curt Lively and Britt Koskella for snail donations, Katelyn Larkin, Andy Thompson, Claire Tucci, and other members of the Neiman lab for help with snail maintenance and data collection, Bennett Brown, Steve Hendrix, and Joel Sharbrough for helpful discussions, and Andrew Forbes, Steve Hendrix, Hang Lim, Dorota Paczesniak, and Joel Sharbrough for comments on an earlier version of the manuscript. We also acknowledge John Endler, F. Xavier Picó, Martin Haase, and an anonymous reviewer for very constructive criticism and suggestions that much improved the manuscript. This research was funded by the Secondary Student Training Program at the University of Iowa, the Iowa Center for Research by Undergraduates, and by the Research Council of Norway.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neiman, M., Warren, D., Rasmussen, B. et al. Complex consequences of increased density for reproductive output in an invasive freshwater snail. Evol Ecol 27, 1117–1127 (2013). https://doi.org/10.1007/s10682-013-9632-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-013-9632-4