Abstract
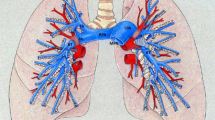
Patient-specific computational models could aid in planning interventions to relieve pulmonary arterial stenoses common in many forms of congenital heart disease. We describe a new approach to simulate blood flow in subject-specific models of the pulmonary arteries that consists of a numerical model of the proximal pulmonary arteries created from three-dimensional medical imaging data with terminal impedance boundary conditions derived from linear wave propagation theory applied to morphometric models of distal vessels. A tuning method, employing numerical solution methods for nonlinear systems of equations, was developed to modify the distal vasculature to match measured pressure and flow distribution data. One-dimensional blood flow equations were solved with a finite element method in image-based pulmonary arterial models using prescribed inlet flow and morphometry-based impedance at the outlets. Application of these methods in a pilot study of the effect of removal of unilateral pulmonary arterial stenosis induced in a pig showed good agreement with experimental measurements for flow redistribution and main pulmonary arterial pressure. Next, these methods were applied to a patient with repaired tetralogy of Fallot and predicted insignificant hemodynamic improvement with relief of the stenosis. This method of coupling image-based and morphometry-based models could enable increased fidelity in pulmonary hemodynamic simulation.






Similar content being viewed by others
References
Alderson P. O., Boonvisut S., McKnight R. C., Jr. Hartman A. F. (1976) Pulmonary perfusion abnormalities and ventilation-perfusion imbalance in children after total repair of tetralogy of Fallot. Circulation 53:332–337
Beard D. A., Bassingthwaighte J. B. (2000) The fractal nature of myocardial blood flow emerges from a whole-organ model of arterial network. J. Vasc. Res. 37:282–296
Broyden C. G. (1965) A class of methods for solving nonlinear simultaneous equations. Math. Comput. 19:577–593
Burrowes K. S., Hunter P. J., Tawhai M. H. (2005) Anatomically-based finite element models of the human pulmonary arterial and venous trees including supernumerary vessels. J. Appl. Physiol. 99:731–738
Dawson C.A., Linehan J.H. (1997) Dynamics of blood flow and pressure-flow relationship. In: Crystal R., J. West, P. Barnes, and E. Weibel (eds.) The Lung: Scientific Foundations. Philadelphia: Lippincott-Raven
Dowdle S. C., Human D. G., Mann M. D. (1990) Pulmonary ventilation and perfusion abnormalities and ventilation perfusion imbalance in children with pulmonary atresia or extreme tetralogy of Fallot. J. Nucl. Med. 31:1276–1279
Figueroa C. A., Vignon-Clementel I. E., Jansen K. E., Hughes T. J. R., Taylor C. A. (2006) A coupled momentum method for modeling blood flow in three-dimensional deformable arteries. Comput. Methods Appl. Mech. Eng. 195:5685–5706
Frank O. (1920) Die elastizität der blutgefässe. Z. Biol. 71:255–272
Gan R. Z., Yen R. T. (1994) Vascular impedance analysis in dog lung with detailed morphometric and elasticity data. J. Appl. Physiol. 77:706–717
Gao J., Huang W., Yen R. T. (2000) Microcirculation impedance analysis in cat lung. J. Biomech. Eng. 122:99–103
Glenny R. W., Robertson H. T. (1995) A computer simulation of pulmonary perfusion in three dimensions. J. Appl. Physiol. 79:357–369
Grant B. J., Paradowski L. J. (1987) Characterization of pulmonary arterial input impedance with lumped parameter models. Am. J. Physiol. 252:H585–593
Huang W., Tian Y., Gao J., Yen R.,T. (1998) Comparison of theory and experiment in pulsatile flow in cat lung. Ann. Biomed. Eng. 26:812–820
Huang W., Yen R. T., McLaurine M., Bledsoe G. (1996) Morphometry of the human pulmonary vasculature. J. Appl. Physiol. 81:2123–2133
Hughes, T. J. R. A Study of the One-Dimensional Theory of Arterial Pulse Propagation. PhD Thesis, U C Berkeley, Berkeley, CA, 1974
Hughes T. J. R., Lubliner J. (1973) On the one-dimensional theory of blood flow in the larger vessels. Math. Biosci. 18:161–170
Ilbawi M. N., Idriss F. S., DeLeon S. Y., Muster A. J., Gidding S. S., Berry T. E., Paul M. H. (1987) Factors that exaggerate the deleterious effects of pulmonary insufficiency on the right ventricle after tetralogy repair. Surgical implications. J. Thorac. Cardiovasc. Surg. 93:36–44
Kassab G. S., Berkley J., Fung Y. C. (1997) Analysis of pig’s coronary arterial blood flow with detailed anatomical data. Ann. Biomed. Eng. 25:204–217
Kassab G. S., Rider C. A., Tang N. J., Fung Y. C. (1993) Morphometry of pig coronary arterial trees. Am. J. Physiol. 265:H350–365
Krenz G. S., Linehan J. H., Dawson C. A. (1992) A fractal continuum model of the pulmonary arterial tree. J. Appl. Physiol. 72:2225–2237
Laganà K., Balossino R., Migliavacca F., Pennati G., Bove E. L., de Leval M. R., Dubini G. (2005) Multiscale modeling of the cardiovascular system: Application to the study of pulmonary and coronary perfusions in the univentricular circulation. J. Biomech. 38:1129–1141
Markl M., Chan F. P., Alley M. T., Wedding K. L., Draney M. T., Elkins C. J., Parker D. W., Wicker R., Taylor C. A., Herfkens R. J., Pelc N. J. (2003) Time-resolved three-dimensional phase-contrast MRI. J. Magn. Reson. Imaging. 17:499–506
McElhinney D. B., Parry A. J., Reddy V. M., Hanley F. L., Stanger P. (1998) Left pulmonary artery kinking caused by outflow tract dilatation after transannular patch repair of tetralogy of Fallot. Ann. Thorac. Surg. 65:1120–1126
Molthen R. C., Karau K. L., Dawson C. A. (2004) Quantitative models of the rat pulmonary arterial tree morphometry applied to hypoxia-induced arterial remodeling. J. Appl. Physiol. 97:2372–2384
Murgo J. P., Westerhof N. (1984) Input impedance of the pulmonary arterial system in normal man. Effects of respiration and comparison to systemic impedance. Circ. Res. 54:666–673
Olufsen M. S. (1999) Structured tree outflow condition for blood flow in larger systemic arteries. Am. J. Physiol. 276:H257–268
Pries A. R., Secomb T. W. (2005) Microvascular blood viscosity in vivo and the endothelial surface layer. Am. J. Physiol. Heart. Circ. Physiol. 289:H2657–2664
Rhodes J., Dave A., Pulling M. C., Geggel R. L., Marx G. R., Fulton D. R., Hijazi Z. M. (1998) Effect of pulmonary artery stenoses on the cardiopulmonary response to exercise following repair of tetralogy of Fallot. Am. J. Cardiol. 81:1217–1219
Seeley B. D., Young D. F. (1976) Effect of geometry on pressure losses across models of arterial stenoses. J. Biomech. 9:439–448
Segers P., Stergiopulos N., Westerhof N., Wouters P., Kolh P., Verdonck P. (2003) Systematic and pulmonary hemodynamics assessed with a lumped-parameter heart-arterial interaction model. J. Eng. Math. 47:185–199
Smith N. P., Pullan A. J., Hunter P. J. (2000) Generation of an anatomically based geometric coronary model. Ann. Biomed. Eng. 28:14–25
Smith N. P., Pullan A. J., Hunter P. J. (2002) An anatomically based model of transient coronary blood flow in the heart. SIAM J. Appl. Math. 62:990–1018
Steele B. N., Olufsen M. S., Taylor C. A. (2007) Fractal network model for simulating abdominal and lower extremity blood flow during resting and exercise conditions. Comput. Methods Biomech. Biomed. Eng.10:39–51
Steele B. N., Wan J., Ku J. P., Hughes T. J. R., Taylor C. A. (2003) In vivo validation of a one-dimensional finite-element method for predicting blood flow in cardiovascular bypass grafts. IEEE Trans. Biomed. Eng. 50:649–656
Taylor M. G. (1959) An experimental determination of the propagation of fluid oscillations in a tube with a visco-elastic wall; together with an analysis of the characteristics required in an electrical analogue. Phys. Med. Biol. 4:63–82
Taylor M. G. (1966) The input impedance of an assembly of randomly branching elastic tubes. Biophys. J. 6:29–51
Taylor M. G. (1966) Wave transmission through an assembly of randomly branching elastic tubes. Biophys. J. 6:697–716
Taylor C. A., Draney M. T., Ku J. P., Parker D., Steele B. N., Wang K., Zarins C. K. (1999) Predictive medicine: Computational techniques in therapeutic decision-making. Comput. Aided Surg. 4:231–247
Tomassoni T. L., Galioto F. M. Jr., Vaccaro P. (1991) Cardiopulmonary exercise testing in children following surgery for tetralogy of Fallot. Am. J. Dis. Child. 145:1290–1293
Vignon-Clementel I. E., Figueroa C. A., Jansen K. E., Taylor C. A. (2006) Outflow boundary conditions for three-dimensional finite element modeling of blood flow and pressure in arteries. Comput. Methods Appl. Mech. Eng. 195:3776–3796
Vignon I. E., Taylor C. A. (2004) Outflow boundary conditions for one-dimensional finite element modeling of blood flow and pressure waves in arteries. Wave Motion 39:361–374
Wan J., Steele B. N., Spicer S. A., Strohband S., Feijóo G. R., Hughes T. J. R., Taylor C. A. (2002) A one-dimensional finite element method for simulation-based medical planning for cardiovascular disease. Comput. Methods Biomech. Biomed. Eng. 5:195–206
Wang K.C., Dutton R.W., Taylor C.A. (1999) Level sets for vascular model construction in computational hemodynamics. IEEE Eng. Med. Biol. 18:33–39
Wilson N. M., Wang K. C., Dutton R. W., Taylor C. A. (2001) A software framework for creating patient specific geometric models from medical imaging data for simulation based medical planning of vascular surgery. Lect. Notes Comput. Sci. 2208:449–456
Womersley J. R. (1957) Oscillatory flow in arteries: The constrained elastic tube as a model of arterial flow and pulse transmission. Phys. Med. Biol. 2:178–187
Zhuang F. Y., Fung Y. C., Yen R. T. (1983) Analysis of blood flow in cat’s lung with detailed anatomical and elasticity data. J. Appl. Physiol. 55:1341–1348
Acknowledgments
The authors wish to thank Dr. Mary T. Draney and the staff of the Richard M. Lucas Center for Magnetic Resonance Spectroscopy and Imaging for assistance with the porcine experiment. This work was supported by the Vera Moulton Wall Center for Pulmonary Vascular Disease and the National Science Foundation under Grant No. 0205741. Ryan Spilker was supported by the Benchmark Capital Fellowship in Congenital Cardiovascular Bioengineering at Stanford University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spilker, R.L., Feinstein, J.A., Parker, D.W. et al. Morphometry-Based Impedance Boundary Conditions for Patient-Specific Modeling of Blood Flow in Pulmonary Arteries. Ann Biomed Eng 35, 546–559 (2007). https://doi.org/10.1007/s10439-006-9240-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-006-9240-3