Abstract
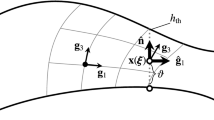
Patient-specific arterial fluid–structure interaction computations, including aorta computations, require an estimation of the zero-stress state (ZSS), because the image-based arterial geometries do not come from a ZSS. We have earlier introduced a method for estimation of the element-based ZSS (EBZSS) in the context of finite element discretization of the arterial wall. The method has three main components. 1. An iterative method, which starts with a calculated initial guess, is used for computing the EBZSS such that when a given pressure load is applied, the image-based target shape is matched. 2. A method for straight-tube segments is used for computing the EBZSS so that we match the given diameter and longitudinal stretch in the target configuration and the “opening angle.” 3. An element-based mapping between the artery and straight-tube is extracted from the mapping between the artery and straight-tube segments. This provides the mapping from the arterial configuration to the straight-tube configuration, and from the estimated EBZSS of the straight-tube configuration back to the arterial configuration, to be used as the initial guess for the iterative method that matches the image-based target shape. Here we present the version of the EBZSS estimation method with isogeometric wall discretization. With isogeometric discretization, we can obtain the element-based mapping directly, instead of extracting it from the mapping between the artery and straight-tube segments. That is because all we need for the element-based mapping, including the curvatures, can be obtained within an element. With NURBS basis functions, we may be able to achieve a similar level of accuracy as with the linear basis functions, but using larger-size and much fewer elements. Higher-order NURBS basis functions allow representation of more complex shapes within an element. To show how the new EBZSS estimation method performs, we first present 2D test computations with straight-tube configurations. Then we show how the method can be used in a 3D computation where the target geometry is coming from medical image of a human aorta.




































Similar content being viewed by others
References
Torii R, Oshima M, Kobayashi T, Takagi K, Tezduyar TE (2004) Computation of cardiovascular fluid–structure interactions with the DSD/SST method. In: Proceedings of the 6th world congress on computational mechanics (CD-ROM), Beijing
Torii R, Oshima M, Kobayashi T, Takagi K, Tezduyar TE (2004) Influence of wall elasticity on image-based blood flow simulations. Trans Jpn Soc Mech Eng Ser A 70:1224–1231. doi:10.1299/kikaia.70.1224 (in Japanese)
Torii R, Oshima M, Kobayashi T, Takagi K, Tezduyar TE (2006) Computer modeling of cardiovascular fluid–structure interactions with the deforming-spatial-domain/stabilized space–time formulation. Comput Methods Appl Mech Eng 195:1885–1895. doi:10.1016/j.cma.2005.05.050
Torii R, Oshima M, Kobayashi T, Takagi K, Tezduyar TE (2006) Fluid–structure interaction modeling of aneurysmal conditions with high and normal blood pressures. Comput Mech 38:482–490. doi:10.1007/s00466-006-0065-6
Tezduyar TE (1992) Stabilized finite element formulations for incompressible flow computations. Adv Appl Mech 28:1–44. doi:10.1016/S0065-2156(08)70153-4
Tezduyar TE (2003) Computation of moving boundaries and interfaces and stabilization parameters. Int J Numer Methods Fluids 43:555–575. doi:10.1002/fld.505
Hughes TJR, Liu WK, Zimmermann TK (1981) Lagrangian–Eulerian finite element formulation for incompressible viscous flows. Comput Methods Appl Mech Eng 29:329–349
Bazilevs Y, Calo VM, Zhang Y, Hughes TJR (2006) Isogeometric fluid–structure interaction analysis with applications to arterial blood flow. Comput Mech 38:310–322
Bazilevs Y, Calo VM, Tezduyar TE, Hughes TJR (2007) YZ\(\beta \) discontinuity-capturing for advection-dominated processes with application to arterial drug delivery. Int J Numer Methods Fluids 54:593–608. doi:10.1002/fld.1484
Bazilevs Y, Calo VM, Hughes TJR, Zhang Y (2008) Isogeometric fluid–structure interaction: theory, algorithms, and computations. Comput Mech 43:3–37
Isaksen JG, Bazilevs Y, Kvamsdal T, Zhang Y, Kaspersen JH, Waterloo K, Romner B, Ingebrigtsen T (2008) Determination of wall tension in cerebral artery aneurysms by numerical simulation. Stroke 39:3172–3178
Bazilevs Y, Gohean JR, Hughes TJR, Moser RD, Zhang Y (2000) Patient-specific isogeometric fluid-structure interaction analysis of thoracic aortic blood flow due to implantation of the Jarvik 2000 left ventricular assist device. Comput Methods Appl Mech Eng 198(2009):3534–3550
Bazilevs Y, Hsu M-C, Benson D, Sankaran S, Marsden A (2009) Computational fluid–structure interaction: methods and application to a total cavopulmonary connection. Comput Mech 45:77–89
Bazilevs Y, Hsu M-C, Zhang Y, Wang W, Liang X, Kvamsdal T, Brekken R, Isaksen J (2010) A fully-coupled fluid–structure interaction simulation of cerebral aneurysms. Comput Mech 46:3–16
Sugiyama K, Ii S, Takeuchi S, Takagi S, Matsumoto Y (2010) Full Eulerian simulations of biconcave neo-Hookean particles in a Poiseuille flow. Comput Mech 46:147–157
Bazilevs Y, Hsu M-C, Zhang Y, Wang W, Kvamsdal T, Hentschel S, Isaksen J (2010) Computational fluid–structure interaction: methods and application to cerebral aneurysms. Biomech Model Mechanobiol 9:481–498
Bazilevs Y, del Alamo JC, Humphrey JD (2010) From imaging to prediction: emerging non-invasive methods in pediatric cardiology. Progr Pediatr Cardiol 30:81–89
Hsu M-C, Bazilevs Y (2011) Blood vessel tissue prestress modeling for vascular fluid–structure interaction simulations. Finite Elem Anal Des 47:593–599
Yao JY, Liu GR, Narmoneva DA, Hinton RB, Zhang Z-Q (2012) Immersed smoothed finite element method for fluid–structure interaction simulation of aortic valves. Comput Mech 50:789–804
Bazilevs Y, Takizawa K, Tezduyar TE (2013) Computational fluid–structure tnteraction: methods and applications. Wiley, Cambridge ISBN 978-0470978771
Bazilevs Y, Takizawa K, Tezduyar TE (2013) Challenges and directions in computational fluid–structure interaction. Math Models Methods Appl Sci 23:215–221. doi:10.1142/S0218202513400010
Long CC, Marsden AL, Bazilevs Y (2013) Fluid–structure interaction simulation of pulsatile ventricular assist devices. Comput Mech 52:971–981. doi:10.1007/s00466-013-0858-3
Esmaily-Moghadam M, Bazilevs Y, Marsden AL (2013) A new preconditioning technique for implicitly coupled multidomain simulations with applications to hemodynamics. Comput Mech 52:1141–1152. doi:10.1007/s00466-013-0868-1
Long CC, Esmaily-Moghadam M, Marsden AL, Bazilevs Y (2014) Computation of residence time in the simulation of pulsatile ventricular assist devices. Comput Mech 54:911–919. doi:10.1007/s00466-013-0931-y
Yao J, Liu GR (2014) A matrix-form GSM–CFD solver for incompressible fluids and its application to hemodynamics. Comput Mech 54:999–1012. doi:10.1007/s00466-014-0990-8
Long CC, Marsden AL, Bazilevs Y (2014) Shape optimization of pulsatile ventricular assist devices using FSI to minimize thrombotic risk. Comput Mech 54:921–932. doi:10.1007/s00466-013-0967-z
Hsu M-C, Kamensky D, Bazilevs Y, Sacks MS, Hughes TJR (2014) Fluid–structure interaction analysis of bioprosthetic heart valves: significance of arterial wall deformation. Comput Mech 54:1055–1071. doi:10.1007/s00466-014-1059-4
Hsu M-C, Kamensky D, Xu F, Kiendl J, Wang C, Wu MCH, Mineroff J, Reali A, Bazilevs Y, Sacks MS (2015) Dynamic and fluid–structure interaction simulations of bioprosthetic heart valves using parametric design with T-splines and fung-type material models. Comput Mech 55:1211–1225. doi:10.1007/s00466-015-1166-x
Kamensky D, Hsu M-C, Schillinger D, Evans JA, Aggarwal A, Bazilevs Y, Sacks MS, Hughes TJR (2015) An immersogeometric variational framework for fluid–structure interaction: application to bioprosthetic heart valves. Comput Methods Appl Mech Eng 284:1005–1053
Tezduyar TE, Sathe S, Cragin T, Nanna B, Conklin BS, Pausewang J, Schwaab M (2007) Modeling of fluid–structure interactions with the space–time finite elements: arterial fluid mechanics. Int J Numer Methods Fluids 54:901–922. doi:10.1002/fld.1443
Tezduyar TE, Sathe S, Schwaab M, Conklin BS (2008) Arterial fluid mechanics modeling with the stabilized space–time fluid–structure interaction technique. Int J Numer Methods Fluids 57:601–629. doi:10.1002/fld.1633
Tezduyar TE, Schwaab M, Sathe S (2009) Sequentially-coupled arterial fluid–structure tnteraction (SCAFSI) technique. Comput Methods Appl Mech Eng 198:3524–3533. doi:10.1016/j.cma.2008.05.024
Takizawa K, Christopher J, Tezduyar TE, Sathe S (2010) Space–time finite element computation of arterial fluid–structure interactions with patient-specific data. Int J Numer Methods Biomed Eng 26:101–116. doi:10.1002/cnm.1241
Tezduyar TE, Takizawa K, Moorman C, Wright S, Christopher J (2010) Multiscale sequentially-coupled arterial FSI technique. Comput Mech 46:17–29. doi:10.1007/s00466-009-0423-2
Takizawa K, Moorman C, Wright S, Christopher J, Tezduyar TE (2010) Wall shear stress calculations in space–time finite element computation of arterial fluid–structure interactions. Comput Mech 46:31–41. doi:10.1007/s00466-009-0425-0
Takizawa K, Moorman C, Wright S, Purdue J, McPhail T, Chen PR, Warren J, Tezduyar TE (2011) Patient-specific arterial fluid–structure interaction modeling of cerebral aneurysms. Int J Numer Methods Fluids 65:308–323. doi:10.1002/fld.2360
Tezduyar TE, Takizawa K, Brummer T, Chen PR (2011) Space–time fluid–structure interaction modeling of patient-specific cerebral aneurysms. Int J Numer Methods Biomed Eng 27:1665–1710. doi:10.1002/cnm.1433
Takizawa K, Brummer T, Tezduyar TE, Chen PR (2012) A comparative study based on patient-specific fluid–structure interaction modeling of cerebral aneurysms. J Appl Mech 79:010908. doi:10.1115/1.4005071
Takizawa K, Schjodt K, Puntel A, Kostov N, Tezduyar TE (2012) Patient-specific computer modeling of blood flow in cerebral arteries with aneurysm and stent. Comput Mech 50:675–686. doi:10.1007/s00466-012-0760-4
Takizawa K, Schjodt K, Puntel A, Kostov N, Tezduyar TE (2013) Patient-specific computational analysis of the influence of a stent on the unsteady flow in cerebral aneurysms. Comput Mech 51:1061–1073. doi:10.1007/s00466-012-0790-y
Takizawa K, Bazilevs Y, Tezduyar TE, Long CC, Marsden AL, Schjodt K (2014) ST and ALE-VMS methods for patient-specific cardiovascular fluid mechanics modeling. Math Models Methods Appl Sci 24:2437–2486. doi:10.1142/S0218202514500250
Takizawa K, Bazilevs Y, Tezduyar TE, Long CC, Marsden AL, Schjodt K (2014) Patient-specific cardiovascular fluid mechanics analysis with the ST and ALE-VMS methods. In: Idelsohn SR (ed) Numerical simulations of coupled problems in engineering of computational methods in applied sciences. Springer, Basel, pp 71–102
Takizawa K, Tezduyar TE, Buscher A, Asada S (2014) Space–time interface-tracking with topology change (ST-TC). Comput Mech 54:955–971. doi:10.1007/s00466-013-0935-7
Takizawa K (2014) Computational engineering analysis with the new-generation space–time methods. Comput Mech 54:193–211. doi:10.1007/s00466-014-0999-z
Takizawa K, Tezduyar TE, Buscher A, Asada S (2014) Space–time fluid mechanics computation of heart valve models. Comput Mech 54:973–986. doi:10.1007/s00466-014-1046-9
Suito H, Takizawa K, Huynh VQH, Sze D, Ueda T (2014) FSI analysis of the blood flow and geometrical characteristics in the thoracic aorta. Comput Mech 54:1035–1045. doi:10.1007/s00466-014-1017-1
Takizawa K, Torii R, Takagi H, Tezduyar TE, Xu XY (2014) Coronary arterial dynamics computation with medical-image-based time-dependent anatomical models and element-based zero-stress state estimates. Comput Mech 54:1047–1053. doi:10.1007/s00466-014-1049-6
Takizawa K, Tezduyar TE (2012) Space–time fluid–structure interaction methods. Math Models Methods Appl Sci 22(supp02):1230001. doi:10.1142/S0218202512300013
Takizawa K, Bazilevs Y, Tezduyar TE, Hsu M-C, Øiseth O, Mathisen KM, Kostov N, McIntyre S (2014) Engineering analysis and design with ALE-VMS and space–time methods. Arch Comput Methods Eng 21:481–508. doi:10.1007/s11831-014-9113-0
Takizawa K, Bazilevs Y, Tezduyar TE, Hsu MC, Øiseth O, Mathisen KM, Kostov N, McIntyre S (2014) Computational engineering analysis and design with ALE-VMS and ST methods. In: Idelsohn SR (ed) Numerical simulations of coupled problems in engineering of computational methods in applied sciences. Springer, Basel
Bazilevs Y, Takizawa K, Tezduyar TE (2015) New directions and challenging computations in fluid dynamics modeling with stabilized and multiscale methods. Math Models Methods Appl Sci 25:2217–2226. doi:10.1142/S0218202515020029
Takizawa K, Tezduyar TE (2016) New directions in space–time computational methods. In: Bazilevs Y, Takizawa K (eds) Advances in computational fluid–structure interaction and flow simulation: new methods and challenging computations, modeling and simulation in science, engineering and technology. Springer, New York, pp 159–178
Takizawa K, Bazilevs Y, Tezduyar TE (2012) Space–time and ALE-VMS techniques for patient-specific cardiovascular fluid–structure interaction modeling. Arch Comput Methods Eng 19:171–225. doi:10.1007/s11831-012-9071-3
Tezduyar TE, Cragin T, Sathe S, Nanna B (2007) FSI computations in arterial fluid mechanics with estimated zero-pressure arterial geometry. In: Onate E, Garcia J, Bergan P, Kvamsdal T (eds) Marine 2007. CIMNE, Barcelona
Takizawa K, Takagi H, Tezduyar TE, Torii R (2014) Estimation of element-based zero-stress state for arterial FSI computations. Comput Mech 54:895–910. doi:10.1007/s00466-013-0919-7
Takizawa K, Tezduyar TE, Sasaki T (2016) Estimation of element-based zero-stress state in arterial FSI computations with isogeometric wall discretization. Springer, New York
Borden MJ, Scott MA, Evans JA, Hughes T (2011) Isogeometric finite element data structures based on Bézier extraction of NURBS. Int J Numer Methods Eng 87:15–47
Acknowledgments
This work was supported in part by JST-CREST; Grant-in-Aid for Scientific Research (S) 26220002 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT); Rice–Waseda research agreement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takizawa, K., Tezduyar, T.E. & Sasaki, T. Aorta modeling with the element-based zero-stress state and isogeometric discretization. Comput Mech 59, 265–280 (2017). https://doi.org/10.1007/s00466-016-1344-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00466-016-1344-5