Abstract
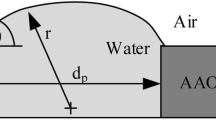
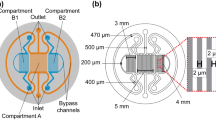
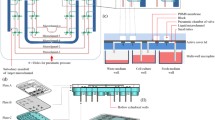
We have developed an on-chip CO2 incubation system based on mass/heat transfer from aqueous solutions of bicarbonate source to cell culture media through a permeable poly(dimethylsiloxane) (PDMS) wall. Heating a carbonate-buffered bicarbonate solution successfully regulated CO2 generation without any feedback control. Because a microfluidic cell culture chip with the incubation system does not require an external chamber or gas supply, the entire microfluidic cell culture setup becomes pocket sized. Using 5 ml of 0.8 M sodium bicarbonate with 65 mM sodium carbonate as the water jacket, the chip maintained the temperature, osmolality, and pH of 750 μl cell culture medium within physiological levels when the chip was placed on a 37°C surface. The osmolality shift and pCO2 of the media reservoir stabilized within <5 mmol/kg and 5.0 ± 1.0% over at least 9 days. The incubation capabilities were demonstrated through microfluidic culture of COS-7 epithelial cells under an inverted microscope for 17 days.







Similar content being viewed by others
References
Addae-Mensah KA, Cheung YK, Fekete V, Rendely MS, Sia SK (2010) Actuation of elastomeric microvalves in point-of-care settings using handheld, battery-powered instrumentation. Lab Chip 10(12):1618–1622. doi:10.1039/c002349c
Barbulovic-Nad I, Au SH, Wheeler AR (2010) A microfluidic platform for complete mammalian cell culture. Lab Chip 10(12):1536–1542. doi:10.1039/c002147d
Choi YH, Son SU, Lee SS (2006) Micro cell incubator with on-chip integrated carbon dioxide generator as a self pH controller. Key Eng Mater 326–328:879–882. doi:10.4028/0-87849-415-4.879
DSMZ (2011) COS-7. http://www.dsmz.de/human_and_animal_cell_lines/info.php?dsmz_nr=60. Accessed 19 Jul 2011
Filley GF, Kindig NB (1985) Carbicarb, an alkalinizing ion-generating agent of possible clinical usefulness. Trans Am Clin Climatol Assoc 96:141–153
Forry SP, Locascio LE (2011) On-chip CO2 control for microfluidic cell culture. Lab Chip 11(23):4041–4046
Freshney RI (2005) Culture of animal cells, 5th edn. Wiley, New Jersey
Futai N, Gu W, Song JW, Takayama S (2006) Handheld recirculation system and customized media for microfluidic cell culture. Lab Chip 6(1):149–154. doi:10.1039/b510901a
Gu W, Zhu X, Futai N, Cho BS, Takayama S (2004) Computerized microfluidic cell culture using elastomeric channels and Braille displays. Proc Natl Acad Sci USA 101(45):15861–15866. doi:10.1073/pnas.0404353101
Ham RG, Puck TT (1962) A regulated incubator controlling CO2 concentration, humidity and temperature for use in animal cell culture. Proc Soc Exp Biol Med 111:67–71
Heo YS, Cabrera LM, Song JW, Futai N, Tung YC, Smith GD, Takayama S (2007) Characterization and resolution of evaporation-mediated osmolality shifts that constrain microfluidic cell culture in poly(dimethylsiloxane) devices. Anal Chem 79(3):1126–1134. doi:10.1021/ac061990v
Hulme SE, Shevkoplyas SS, Whitesides GM (2009) Incorporation of prefabricated screw, pneumatic, and solenoid valves into microfluidic devices. Lab Chip 9(1):79–86. doi:10.1039/b809673b
Kane BJ, Zinner MJ, Yarmush ML, Toner M (2006) Liver-specific functional studies in a microfluidic array of primary mammalian hepatocytes. Anal Chem 78(13):4291–4298. doi:10.1021/ac051856v
Kimura H, Yamamoto T, Sakai H, Sakai Y, Fujii T (2008) An integrated microfluidic system for long-term perfusion culture and on-line monitoring of intestinal tissue models. Lab Chip 8(5):741–746. doi:10.1039/b717091b
Korin N, Bransky A, Khoury M, Dinnar U, Levenberg S (2009) Design of well and groove microchannel bioreactors for cell culture. Biotechnol Bioeng 102(4):1222–1230. doi:10.1002/bit.22153
Merkel TC, Bondar VI, Nagai K, Freeman BD, Pinnau I (2000) Gas sorption, diffusion, and permeation in poly(dimethylsiloxane). J Polym Sci B Polym Phys 38(3):415–434. doi:10.1002/(sici)1099-0488(20000201)38:3<415:aid-polb8>3.0.co;2-z
Miller RG, Phillips RA (1969) Separation of cells by velocity sedimentation. J Cell Phyisiol 73(3):191–201. doi:10.1002/jcp.1040730305
Plummer L, Busenberg E (1982) The solubilities of calcite, aragonite and vaterite in CO2–H2O solutions between 0 and 90°C, and an evaluation of the aqueous model for the system CaCO3–CO2–H2O. Geochim Cosmochim Acta 46(6):1011–1040. doi:10.1016/0016-7037(82)90056-4
Polinkovsky M, Gutierrez E, Levchenko A, Groisman A (2009) Fine temporal control of the medium gas content and acidity and on-chip generation of series of oxygen concentrations for cell cultures. Lab Chip 9(8):1073–1084. doi:10.1039/b816191g
Roth V (2006) Doubling time. http://www.doubling-time.com/compute.php. Accessed 28 May 2011
Severinghaus JW, Bradley AF (1958) Electrodes for blood pO2 and pCO2 determination. J Appl Physiol 13(3):515–520
Waymouth C (1970) Osmolality of mammalian blood and of media for culture of mammalian cells. In Vitro 6(2):109–127. doi:10.1007/BF02616113
Xu ZR, Yang CG, Liu CH, Zhou Z, Fang J, Wang JH (2010) An osmotic micro-pump integrated on a microfluidic chip for perfusion cell culture. Talanta 80(3):1088–1093. doi:10.1016/j.talanta.2009.08.031
Acknowledgments
This research was supported by Global Environment Research Fund by the Ministry of the Environment Japan (C-0803). We also thank Dr. Katsumi Mochitate at the National Institute for Environmental Studies of Japan and Prof. Shuichi Takayama at the University of Michigan for helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takano, A., Tanaka, M. & Futai, N. On-chip CO2 incubation for pocket-sized microfluidic cell culture. Microfluid Nanofluid 12, 907–915 (2012). https://doi.org/10.1007/s10404-011-0925-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-011-0925-z