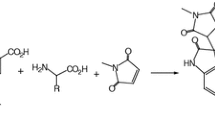
Abstract
The 1,3-dipolar cycloaddition of stable isatin ketonitrone with various dipolarophiles has been conducted under classical, ionic liquid, and solvent-free conditions to give novel spiro[oxindole-isoxazolidine] derivatives with similar diastereoselectivity. In the presence of the ionic liquid 1-butyl-3-methylimidazolium bromide highly diastereoselective and regioselective cyclocondensation products were obtained in good to excellent yields in the absence of any catalyst. The reaction workup was simple and the ionic liquid was easily recovered from the reaction and reused.
Graphical abstract



Similar content being viewed by others
References
Kissane M, Lawrence SE, Maguire AR (2010) Tetrahedron 66:4564
Grigor’ev IA (2008) In: Feuer H (ed), Nitrile oxides, nitrones, and nitronates in organic synthesis: novel strategies in synthesis, 2nd edn. Wiley, Hoboken
Gothelf KV, Jorgensen KA (1998) Chem Rev 98:863
Torssell KGB (1988) Nitrile oxides, nitrones, and nitronates in organic synthesis: novel strategies in synthesis. VCH, New York
Tufariello JJ (1984) In: Padwa A (ed), 1,3-Dipolar cycloaddition chemistry. Wiley, New York
Macdonald JM, Horsley HT, Ryan JH, Saubern S, Holmes AB (2008) Org Lett 10:4227
Voinov MA, Shevelev TG, Rybalova TV, Gatilov YV, Pervukhina NV, Burdukov AB, Grigor’ev IA (2007) Organometallics 26:1607
Pfeiffer JY, Beauchemin AM (2009) J Org Chem 74:8381
Fischer R, Hyrosova E, Fisera L, Hametner C, Cyranski MK (2005) Chem Pap 59:275
Tomioka Y, Nagahiro C, Nomura Y, Maruoka H (2003) J Heterocycl Chem 40:121
Torrente S, Noya B, Branchadell V, Alonso R (2003) J Org Chem 68:4772
Hulsbos E, Marcus J, Brussee J, van der Gen A (1997) Tetrahedron Asymmetry 8:1061
Franco S, Merchan FL, Merino P, Tejero T (1995) Synth Commun 25:2275
Black DSC, Johnstone LM (1984) Aust J Chem 37:117
Exner O (1951) Collect Czech Chem Commun 16:258
Suman Reddy Y, Kadigachalam P, Doddi VR, Vankar YD (2009) Tetrahedron Lett 50:5827
Tacconi G, Righetti PP, Desimoni G (1980) J Prakt Chem 322:679
Aurich HG, Weiss W (1976) Tetrahedron 32:159
Takeuchi Y, Furusaki F (1977) Adv Heterocycl Chem 21:207
Frederickson M (1997) Tetrahedron 53:403
Mzengeza S, Whitney RA (1988) J Org Chem 53:4074
Mzengeza S, Yang CM, Whitney RA (1987) J Am Chem Soc 109:276
Kasahara K, Iida H, Kibayashi C (1989) J Org Chem 54:2225
Iida H, Kasahara K, Kibayashi C (1986) J Am Chem Soc 108:4647
Ooi H, Urushibara A, Esumi T, Iwabuchi Y, Hatakeyama S (2001) Org Lett 3:953
Rong J, Roselt P, Plavec J, Chattopadhyaya J (1994) Tetrahedron 50:4921
Damavandy JA, Mehrdad M (1990) J Sci Islamic Repub Iran 1:96
Bigdeli MA, Mehrdad M (1991) Iran J Chem Chem Eng 10:101
Bigdeli MA, Mehrdad M (1993) J Sci Islamic Repub Iran 4:109
Jadidi K, Ghahremanzadeh R, Mehrdad M, Ghanbari M, Arvin-Nezhad H (2008) Monatsh Chem 139:277
Jadidi K, Gharemanzadeh R, Mehrdad M, Darabi HR, Khavasi HR, Asgari D (2008) Ultrason Sonochem 15:124
Jadidi K, Ghahremanzadeh R, Bazgir A (2009) Tetrahedron 65:2005
Anastas PT, Kirchhoff MM (2002) Acc Chem Res 35:686
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehrdad, M., Faraji, L., Jadidi, K. et al. A regioselective and diastereoselective synthesis of new spiro-isoxazolidines via 1,3-dipolar cycloaddition of stable isatin ketonitrone and various dipolarophiles. Monatsh Chem 142, 917–921 (2011). https://doi.org/10.1007/s00706-011-0518-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-011-0518-2