Abstract
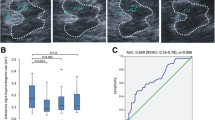
In patients with Wilson’s disease (WD) transcranial brain sonography typically reveals areas of increased echogenicity (hyperechogenicity) of the lenticular nucleus (LN). Correlation with T2-hypointensity on magnetic resonance images suggested that LN hyperechogenicity in WD is caused by trace metal accumulation. Accumulation of both, copper and iron, in the brain of WD patients has been reported. The present study was designed to elucidate whether LN hyperechogenicity in WD reflects accumulation of copper or iron. Post-mortem brains of 15 WD patients and one non-WD subject were studied with ultrasonography in an investigator-blinded fashion. LN hyperechogenicity was measured planimetrically by manual tracing as well as using digitized image analysis. The putaminal copper content was determined in samples of 11 WD brains and the non-WD brains using inductively coupled plasma mass spectrometry, and iron content was assessed using flame atomic absorption spectroscopy. LN was normal on ultrasonography only in the non-WD brain, but abnormal (hyperechogenic) in all WD brains. Digitized image analysis measures of LN hyperechogenicity and, by trend, manual measures correlated with putaminal copper content (Pearson test; digitized: r = 0.77, p = 0.04; manual: r = 0.57, p = 0.051) but not with iron content (each, p > 0.18). LN hyperechogenicity measures were unrelated to age at death of patients, age at onset of WD, WD duration, age of brain specimen, serum copper or serum ceruloplasmin (each, p > 0.1). We conclude that LN hyperechogenicity in WD reflects copper, but not iron accumulation. Further studies are warranted to elucidate the use of transcranial brain sonography for monitoring therapeutic effects of chelating agents in WD patients.


Similar content being viewed by others
References
Becker G, Berg D, Rausch WD, Lange HK, Riederer P, Reiners K (1999) Increased tissue copper and manganese content in the lentiform nucleus in primary adult-onset dystonia. Ann Neurol 46:260–263
Becker G, Berg D, Francis M, Naumann M (2001) Evidence for disturbances of copper metabolism in dystonia: from the image towards a new concept. Neurology 57:2290–2294
Berg D, Hoggenmüller U, Hofmann E et al (2000) The basal ganglia in haemochromatosis. Neuroradiology 42:9–13
Blahuta J, Soukup T, Jelinkova M et al (2013) A new program for highly reproducible automatic evaluation of the substantia nigra from transcranial sonographic images. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. doi:10.5507/bp.2013.029
Bruehlmeier M, Leenders KL, Vontobel P, Calonder C, Antonini A, Weindl A (2000) Increased cerebral iron uptake in Wilson’s disease: a 52Fe-citrate PET study. J Nucl Med 41:781–787
Brüggemann N, Schneider SA, Sander T, Klein C, Hagenah J (2010) Distinct basal ganglia hyperechogenicity in idiopathic basal ganglia calcification. Mov Disord 25:2661–2664
Brüggemann N, Wuerfel J, Petersen D, Klein C, Hagenah J, Schneider SA (2011) Idiopathic NBIA—clinical spectrum and transcranial sonography findings. Eur J Neurol 18:e58–e59
Eicke M, Briner J, Willi U, Uehlinger J, Boltshauser E (1992) Symmetrical thalamic lesions in infants. Arch Dis Child 67:15–19
Hare DJ, Gerlach M, Riederer P (2012) Considerations for measuring iron in post-mortem tissue of Parkinson’s disease patients. J Neural Transm 119:1515–1521
Hayashi H, Hattori A, Tatsumi Y et al (2013) Various copper and iron overload patterns in the livers of patients with Wilson disease and idiopathic copper toxicosis. Med Mol Morphol 46:133–140
Heckmann JM, Eastman RW, De Villiers JC, Hewlett R (1994) Wilson’s disease: neurological and magnetic resonance imaging improvement on zinc treatment. J Neurol Neurosurg Psychiatry 57:1273–1274
Horoupian DS, Sternlieb I, Scheinberg ICH (1988) Neuropathological findings in penicillamine-treated patients with Wilson’s disease. Clin Neuropathol 7:62–67
Kim JM, Ko SB, Kwon SJ et al (2005) Ferrous and ferric iron accumulates in the brain of aged Long-Evans Cinnamon rats, an animal model of Wilson’s disease. Neurosci Lett 382:143–147
King AD, Walshe JM, Kendall BE et al (1996) Cranial MR imaging in Wilson’s disease. AJR Am J Roentgenol 167:1579–1584
Kostić VS, Svetel M, Mijajlović M, Pavlović A, Ječmenica-Lukić M, Kozić D (2012) Transcranial sonography in pantothenate kinase-associated neurodegeneration. J Neurol 259:959–964
Krogias C, Meves S, Schoellhammer M, Gold R, Andrich J (2009) Sonographic detection of bilateral striopallidodentate calcinosis. J Neurol 256:266–267
Litwin T, Gromadzka G, Członkowska A (2012) Gender differences in Wilson’s disease. J Neurol Sci 312:31–35
Litwin T, Gromadzka G, Szpak GM, Jabłonka-Salach K, Bulska E, Członkowska A (2013) Brain metal accumulation in Wilson’s disease. J Neurol Sci 329:55–58
Martínez-Fernández R, Caballol N, Gómez-Choco M (2013) MRI and transcranial sonography findings in Wilson’s disease. Mov Disord 28:740
Meenakshi-Sundaram S, Mahadevan A, Taly AB, Arunodaya GR, Swamy HS, Shankar SK (2008) Wilson’s disease: a clinico-neuropathological autopsy study. J Clin Neurosci 15:409–417
Pfeiffenberger J, Gotthardt DN, Herrmann T et al (2012) Iron metabolism and the role of HFE gene polymorphisms in Wilson disease. Liver Int 32:165–170
Pfeiffer RF (2011) Wilson’s disease. Handb Clin Neurol 100:681–709
Ricciardi MC, Sirimarco G, Vicenzini E et al (2010) Transcranial sonographic findings in Wilson disease. J Ultrasound Med 29:1143–1145
Roberts EA, Schilsky ML; American Association for Study of Liver Diseases (AASLD) (2008) Diagnosis and treatment of Wilson disease: an update. Hepatology 47:2089–2111
Schrag M, Dickson A, Jiffry A, Kirsch D, Vinters HV, Kirsch W (2010) The effect of formalin fixation on the levels of brain transition metals in archived samples. Biometals 23:1123–1127
Sharonova IN, Vorobjev VS, Haas HL (1998) High-affinity copper block of GABAA receptor-meditated currents in acutely isolated cerebellar Purkinje cells of the rat. Eur J Neurosci 10:522–528
Sinha S, Taly AB, Ravishankar S et al (2006) Wilson’s disease: cranial MRI observations and clinical correlation. Neuroradiology 48:613–621
Skjørringe T, Møller LB, Moos T (2012) Impairment of interrelated iron- and copper homeostatic mechanisms in brain contributes to the pathogenesis of neurodegenerative disorders. Front Pharmacol 3:169
Skowronska M, Walter U, Kmiec T, Członkowska A (2013a) Transcranial sonography in mitochondrial membrane protein-associated neurodegeneration. Parkinsonism Relat Disord 19:1061–1063
Skowronska M, Dziezyc K, Członkowska A (2013b) Transcranial sonography in manganese-induced parkinsonism caused by drug abuse. Clin Neuroradiol. doi:10.1007/s00062-013-0256-4
Stys PK, You H, Zamponi GW (2012) Copper-dependent regulation of NMDA receptors by cellular prion protein: implications for neurodegenerative disorders. J Physiol 590:1357–1368
Svetel M, Mijajlović M, Tomić A, Kresojević N, Pekmezović T, Kostić VS (2012) Transcranial sonography in Wilson’s disease. Parkinsonism Relat Disord 18:234–238
Toscano M, Canevelli M, Giacomelli E et al (2011) Transcranial sonography of basal ganglia calcifications in Fahr disease. J Ultrasound Med 30:1032–1033
Van de Loo S, Walter U, Behnke S et al (2010) Reproducibility and diagnostic accuracy of substantia nigra sonography for the diagnosis of Parkinson’s disease. J Neurol Neurosurg Psychiatry 81:1087–1092
Waggoner DJ, Bartnikas TB, Gitlin JD (1999) The role of copper in neurodegenerative disease. Neurobiol Dis 6:221–230
Walter U (2010) Transcranial sonography in brain disorders with trace metal accumulation. Int Rev Neurobiol 90:166–178
Walter U, Krolikowski K, Tarnacka B, Benecke R, Członkowska A, Dressler D (2005) Sonographic detection of basal ganglia lesions in asymptomatic and symptomatic Wilson disease. Neurology 64:1726–1732
Walter U, Dressler D, Lindemann C, Slachevsky A, Miranda M (2008) Transcranial sonography findings in welding-related Parkinsonism in comparison to Parkinson’s disease. Mov Disord 23:141–145
Walter U, Witt R, Wolters A, Wittstock M, Benecke R (2012) Substantia nigra echogenicity in Parkinson’s disease: relation to serum iron and C-reactive protein. J Neural Transm 119:53–57
Walter U, Blitzer A, Benecke R, Grossmann A, Dressler D (2014) Sonographic detection of basal ganglia abnormalities in spasmodic dysphonia. Eur J Neurol 21:349–352
Weiser T, Wienrich M (1996) The effects of copper ions on glutamate receptors in cultured rat cortical neurons. Brain Res 742:211–218
Acknowledgments
This study was supported by the Polish National Science Centre (grant No. NN 402472340).
Conflict of interest
The authors have no financial interests that relate to research covered in this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walter, U., Skowrońska, M., Litwin, T. et al. Lenticular nucleus hyperechogenicity in Wilson’s disease reflects local copper, but not iron accumulation. J Neural Transm 121, 1273–1279 (2014). https://doi.org/10.1007/s00702-014-1184-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-014-1184-4